
[ad_1]
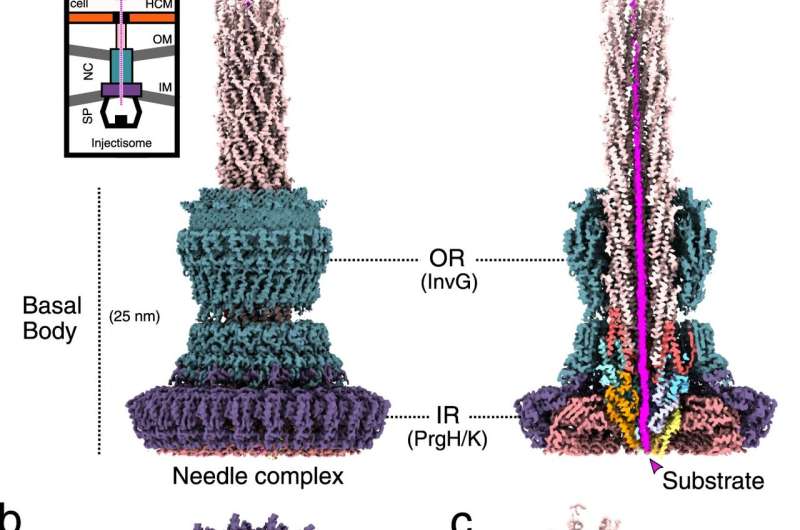
Unsymmetrical cryo-EM (C1) reconstruction of the substrate-engaged needle complex (left) with a vertical cut through the center (right) revealing the substrate shown in magenta throughout the translocation channel. Top left, is a cartoon diagram of the injectisome needle complex in bacterial membranes inner and outer (IM / OM) and in contact with a host cell membrane (HCM). NC: needle complex, SP: sorting platform, OR: outer rings, IR: inner rings. The following colors are used in this figure and all other figures: PrgH1-24: dark purple (hexadecimal color # 6d58ab), PrgK1-24: soft red (# dc888d), InvG1-15 / 16: dark cyan (# 5f919c ), SpaQs: yellow colors (SpaQ1 # FFFF99, SpaQ2 # FFFF66, SpaQ3 # FFCC66, SpaQ4 # CCCC99), SpaPs: blue colors (SpaP1 # 99CCFF, SpaP2 # 66CCFF, SpaP3 #CCCCFF, SpaP4 # 9999FF, SpaP5 # 99FFFF), SpaR: orange (# FF9900), PrgJs: red colors (PrgJ1 # FF3333, PrgJ2-6 # FF6666), PrgI1-72: pale pink (#FFCCCC) and the substrate: magenta (# FF00FF). b Top and bottom views of the C1 board showing the export device (EA) and the substrate. c Cryo-EM map of the filament, inner rod and components of the export device. SpaP1 has been removed to facilitate visualization of the substrate.
For the first time, researchers have revealed the syringe-shaped type III secretion system of Gram-negative bacteria in action. In the research study, published in Nature communications, the group of Thomas Marlovits from the Center for Structural Systems Biology (CSSB) at the DESY campus is providing new insight into how pathogenic bacteria such as Salmonella infect human cells. Visualizing this at the molecular level, the researchers say, helps deepen our understanding of host-pathogen interactions and contributes to the development of new therapies for the treatment of bacterial infections and other diseases.
To infect its human host, many Gram-negative bacteria such as Salmonella or plague bacteria rely on a type III secretion system also known as an injectisome. Each individual bacteria has several syringe-shaped injectisomes protruding from its surface. As the name suggests, injectisomes are responsible for injecting bacterial proteins into human cells. Once inside the cell, these bacterial proteins invade cell tissue and help spread the infection.
The Marlovits group has been studying the molecular mechanisms of the injectisome needle complex for several years. “The needle complex is a cylindrical structure and represents the most prominent part of the injectisome,” explains Marlovits. “It provides bacterial proteins with a fluid passage from the bacterial cytoplasm across multiple membranes directly into human host cells.”
In fact, it has been reported that up to 60 bacterial proteins can pass through the needle complex in a second. While this rapid rate of secretion is beneficial to the bacteria, it hampers the ability of researchers to visualize and understand this essential process. “To help overcome this challenge, we trapped a single bacterial protein inside the needle complex with a trick,” says Sean Miletic, one of the study’s lead authors. Miletic’s colleague Jiri Wald was then able to capture high-resolution images of the protein’s subtle movements inside the needle complex using a cryo electron microscope at CSSB.
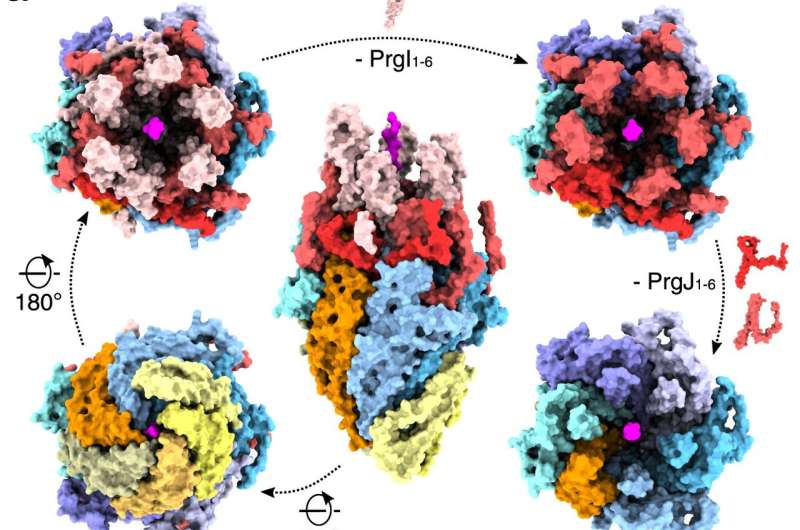
Modular assembly of the EA trapped in the substrate, the inner rod (PrgJ) and the first six PrgI subunits of the helical filament. The individual protein components are indicated in the dotted box below. SptP3x-GFP is displayed in magenta. b Left: view of EA with SpaP1, PrgJ1-2 and PrgI1 removed and the substrate translocation path displayed as a white surface. Right: four discrete sections (portal, channel, atrium and tunnel) of the translocation path are shown with the EM density corresponding to the substrate (threshold: 0.015). Box right: magnification showing the residue surfaces forming hydrophilic and hydrophobic stairs encircling the portal and the channel. Green: hydrophilic; white: neutral; gold: hydrophobic.
The high-resolution images not only provided new insight into the structure of the needle complex, but also confirmed the essential role of its Export Apparatus (EA) which functions as a portal of entry. “The structural elements of EA work together like a gate and a guide, to engage and direct bacterial proteins towards the tip of the needle,” says Dirk Fahrenkamp of the research team, who built an atomic model of the needle complex based on the pictures.
Ultimately, understanding and visualizing the molecular mechanisms used by bacteria to invade human cells at atomic resolution is essential for the development of new therapies capable of fighting infections in targeted and intelligent ways. “While these results enrich our understanding of the type three secretion system, this study is also remarkable in that it was truly a group effort,” says Marlovits. “Not only did each of the first four authors of the article bring their own vital expertise, but the collaborative efforts of the cryo-EM facility and the support of the CSSB Media Kitchen were critical to the success of this study. ”
Researchers develop ‘molecular needle’ using simplified biological system
Sean Miletic et al. The structures of the type III secretion system engaged in the substrate reveal a trigger mechanism for the translocation of unfolded proteins, Nature communications (2021). DOI: 10.1038 / s41467-021-21143-1
Provided by Deutsches Elektronen-Synchrotron
Quote: Researchers first watch injection of Gram-negative bacteria in action (March 10, 2021) retrieved March 10, 2021 from https://phys.org/news/2021-03-injectisome-gram-negative-bacteria -action. html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link