[ad_1]
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which is the virus that causes coronavirus disease 2019 (COVID-19), is associated with high death and hospitalization rates. However, many patients infected with SARS-CoV-2 remain asymptomatic or develop mild symptoms.
In a recent study published on the Preprint Server bioRxiv *, researchers are focusing on the relationship between pre-existing airway neutrophils and SARS-CoV-2 infection to determine the impact neutrophils have on COVID-19.
 Study: Neutrophil-epithelial interactions increase infectivity and pro-inflammatory responses to SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock.com
Study: Neutrophil-epithelial interactions increase infectivity and pro-inflammatory responses to SARS-CoV-2 infection. Image Credit: Kateryna Kon / Shutterstock.com
An overview of neutrophils
Neutrophils are the first and predominant immune cells that are recruited into the respiratory tract in response to a viral infection. Upon arrival, neutrophils release various inflammatory mediators with the aim of rapidly removing the pathogen from the infected area.
Neutrophils are able to recognize sites of infection as well as act as sites of infection which together lead to an acute inflammatory response. A massive uncontrolled inflammatory response, also known as a cytokine storm, has been documented in patients with severe COVID-19.
“Despite their importance in antiviral immunity and the response to viral pathogens, neutrophils have been somewhat neglected for their role in the pathogenesis of SARS-CoV-2 infection.”
About the study
Researchers have developed a novel in vitro model to assess inflammation of the neutrophilic airways in SARS-CoV-2 infection. For this purpose, primary epithelial cells of the human respiratory tract have been isolated from lung tissue.
The present study focused on the isolation of neutrophils from peripheral blood. After their isolation, the purity of the neutrophils was confirmed by flow activated cell sorting (FACS).
Freshly isolated neutrophils were then added to the differentiated airway epithelial cells, followed by infection with SARS-CoV-2 for a total of 4 hours.
Along with these experiments, the researchers also performed immunohistochemistry of major human lung tissues obtained from severe COVID-19 patients.
Study results
Analysis of the pulmonary pathologies of COVID-19 patients confirmed the significant infiltration of inflammatory cells, including neutrophils. Specifically, these post-mortem samples were found to have increased neutrophil elastase (NE) activity compared to neutrophils identified in healthy lung tissue. Elevated neutrophil invasion and epithelial shedding were also observed, suggesting that neutrophils play an important role in SARS-CoV-2 infection.
Peripheral blood samples from COVID-19 patients also showed higher concentrations of pro-inflammatory cytokines that disrupt epithelial permeability in patients with severe disease.
“In addition, marked increases in neutrophils were also observed in bronchiolar alveolar lavage fluids (BAL) of patients admitted to intensive care units (ICUs) compared to patients in intermediate medical units (IMUs) correlating with severity. of disease. “
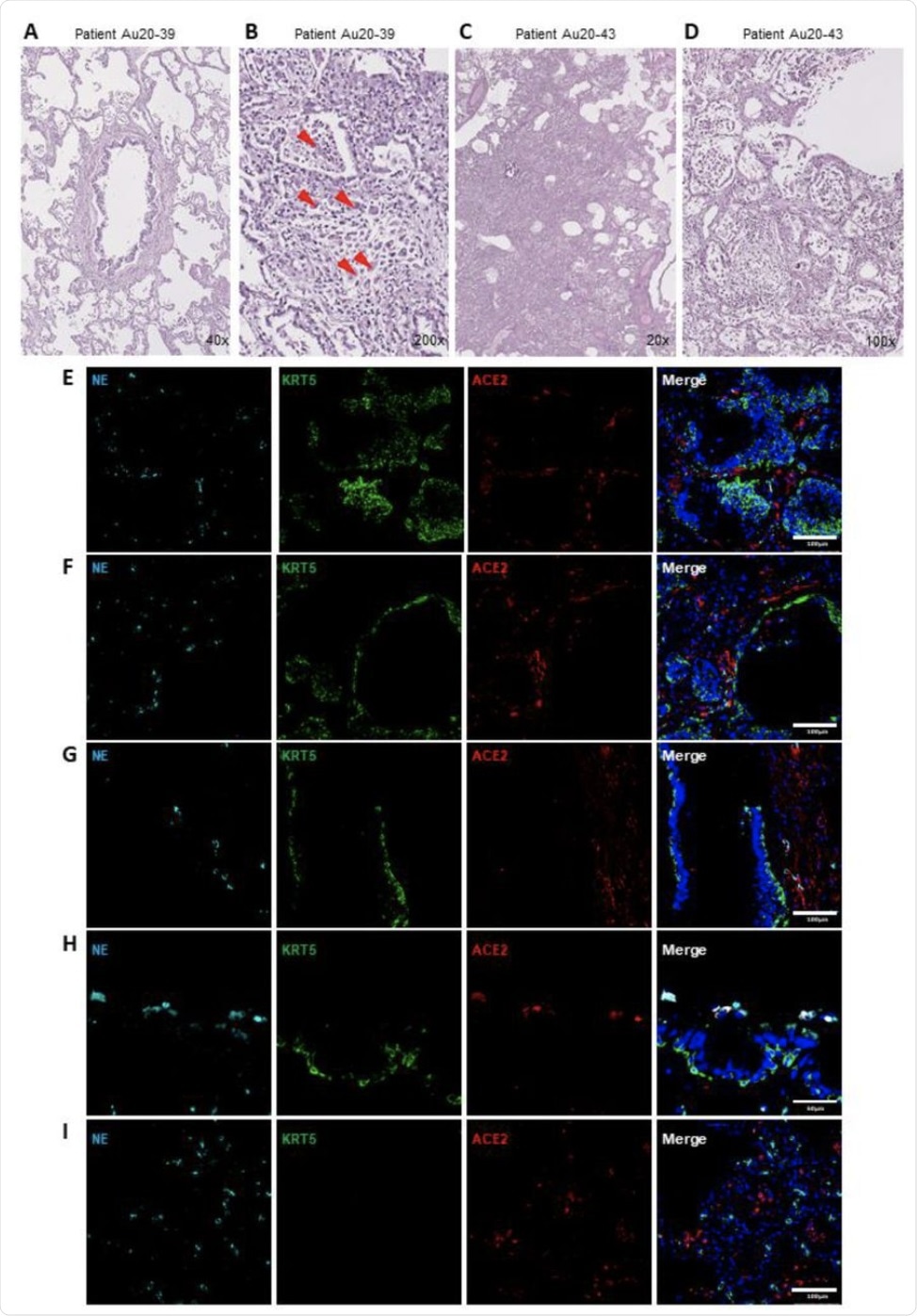 Tissue pathology associated with neutrophils in the human pulmonary airways postmortem COVID19. (AD) Representative images of hematoxylin and eosin (H&E) staining of postmortem tissues of COVID-19 patients showing uneven organized pneumonia centered around a major artery and airway (A ); interstitium focused by a mixed cell infiltrate comprising scattered giant cells (orange arrowheads) (B); diffuse alveolar lesions due to an intense fibro-inflammatory process and rounded air spaces induced by barotrauma (C) and organizing diffuse alveolar lesions with a disposition of fibrin replaced by organized pneumonia, inflammatory cells and edema (D ). (EH) Representative IF images of postmortem COVID-19 tissue probed for NE (cyan), KRT5 (green) and ACE2 (red). Images are highlighted; small airway obstruction resulting from basal cell hyperplasia with the presence of surrounding neutrophils (E); epithelial damage with penetration of neutrophils into the luminal space (F); epithelial excretion, including the basal cell layer with inclusion of neutrophils from the mucous surface (G); breach of neutrophils in the luminal airway space with high activity of neutrophil elastase (H) and diffuse invasion of neutrophils from the alveolar spaces (I). All IF images have nuclei counterstained with DAPI (blue) and scale bars represent 100 µm. All the images are representative of 3 independent regions per donor at least 2 independent donors.
Tissue pathology associated with neutrophils in the human pulmonary airways postmortem COVID19. (AD) Representative images of hematoxylin and eosin (H&E) staining of postmortem tissues of COVID-19 patients showing uneven organized pneumonia centered around a major artery and airway (A ); interstitium focused by a mixed cell infiltrate comprising scattered giant cells (orange arrowheads) (B); diffuse alveolar lesions due to an intense fibro-inflammatory process and rounded air spaces induced by barotrauma (C) and organizing diffuse alveolar lesions with a disposition of fibrin replaced by organized pneumonia, inflammatory cells and edema (D ). (EH) Representative IF images of postmortem COVID-19 tissue probed for NE (cyan), KRT5 (green) and ACE2 (red). Images are highlighted; small airway obstruction resulting from basal cell hyperplasia with the presence of surrounding neutrophils (E); epithelial damage with penetration of neutrophils into the luminal space (F); epithelial excretion, including the basal cell layer with inclusion of neutrophils from the mucous surface (G); breach of neutrophils in the luminal airway space with high activity of neutrophil elastase (H) and diffuse invasion of neutrophils from the alveolar spaces (I). All IF images have nuclei counterstained with DAPI (blue) and scale bars represent 100 µm. All the images are representative of 3 independent regions per donor at least 2 independent donors.
Findings of neutrophilia in the airways of patients with severe COVID-19 have led researchers to assess how inflammation of the neutrophil airways plays a role in SARS-CoV-2 infection. Prior to this determination, the researchers confirmed the expression of angiotensin converting enzyme receptor 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) in their novel in vitro airway epithelium model to confirm its utility in studying the mechanisms of SARS-CoV-2 infection.
“Using this model, we were able to conclude that the presence of neutrophils in the airway epithelium dramatically increases pro-inflammatory responses to SARS-CoV-2, increases the viral load of the airways and decreases the integrity of the respiratory tract. epithelial barrier of the respiratory tract. “
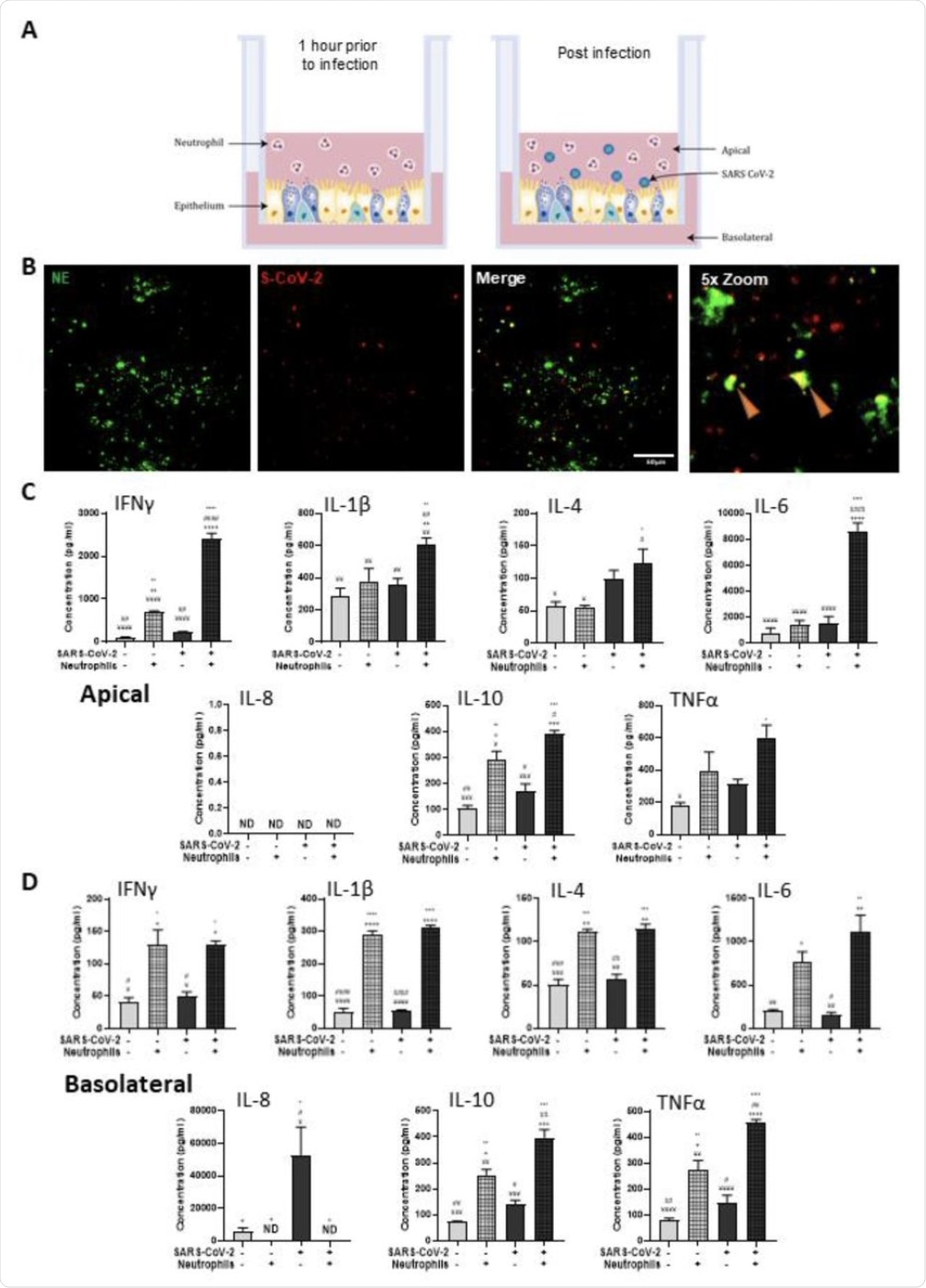 Polarized inflammatory response of neutrophils in co-culture with the epithelium of the human respiratory tract, infected with SARS-CoV-2. (A) Schematic of the in vitro neutrophil airway model designating neutrophils in co-culture with differentiated airway epithelial cells infected with live SARS-CoV-2 virus. (B) Representative IF images of primary human airway epithelial cells differentiated at the air-liquid interface stained with NE (green) and probed by RNAScope for SARS-CoV-2 (red). Scale bars represent 100 μm. Inflammatory profiles of apical (C) and basolateral (D) supernatants collected 4 hours after infection in the neutrophil airway model. Data are expressed as mean ± SEM and significance is determined by analysis of variance (ANOVA) followed by post hoc Tukey analysis. * compared to the uninfected epithelial monoculture, # compared to the uninfected epithelial and neutrophil co-culture, + compared to the infected monoculture epithelial cells, ¥ significant from the infected neutrophil and epithelial co-culture. * p <0.05, ** p <0.01, *** p <0.001, ****, <0.0001 from n = 3 experimental replicates of N = 3 donors.
Polarized inflammatory response of neutrophils in co-culture with the epithelium of the human respiratory tract, infected with SARS-CoV-2. (A) Schematic of the in vitro neutrophil airway model designating neutrophils in co-culture with differentiated airway epithelial cells infected with live SARS-CoV-2 virus. (B) Representative IF images of primary human airway epithelial cells differentiated at the air-liquid interface stained with NE (green) and probed by RNAScope for SARS-CoV-2 (red). Scale bars represent 100 μm. Inflammatory profiles of apical (C) and basolateral (D) supernatants collected 4 hours after infection in the neutrophil airway model. Data are expressed as mean ± SEM and significance is determined by analysis of variance (ANOVA) followed by post hoc Tukey analysis. * compared to the uninfected epithelial monoculture, # compared to the uninfected epithelial and neutrophil co-culture, + compared to the infected monoculture epithelial cells, ¥ significant from the infected neutrophil and epithelial co-culture. * p <0.05, ** p <0.01, *** p <0.001, ****, <0.0001 from n = 3 experimental replicates of N = 3 donors.
Conclusion
“In conclusion, we have developed a model to study neutrophil-epithelial interactions that more closely reflects a in vivo and a more clinically relevant respiratory tract infection in severe cases of COVID-19 than monocultures. “
The results of this study demonstrate that the presence of neutrophils in the airway epithelium leads to the release of pro-inflammatory cytokines mediated by SARS-CoV-2. This acute inflammatory response therefore leads to an increase in the viral load and a decrease in the integrity of the barrier.
“Overall, we make important observations that reveal a key role of neutrophil-epithelial interactions in determining infectivity and outcomes in response to SARS-CoV-2 infection that highlight neutrophils as a potential target for the prevention of severe COVID-19 disease, ”the team adds.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link