
[ad_1]
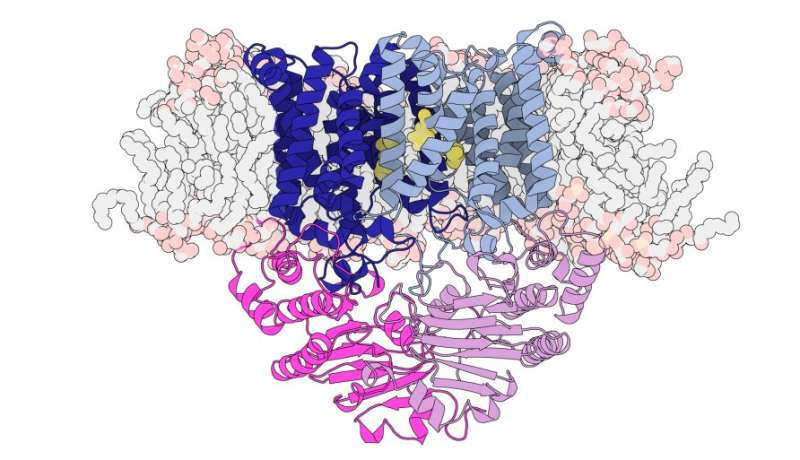
Structure of the manganese transporter S. pneumoniae including transmembrane regions (blue), energy domains (pink) and membrane lipids in pale gray with pale orange heads. Yellow sections represent the position of manganese-specific trigger residues. Credit: Hugo MacDermott-Opeskin, Megan O’Mara and Christopher McDevitt
The invitations, the decorations, the candles and the cake – there are a lot of things to remember when throwing a birthday party for a five year old. One thing we happily take for granted in Australia is that our five year olds are reaching this milestone.
But nearly half a million children worldwide infected with Streptococcus pneumoniae bacteria each year never reach their 5th birthday.
Like COVID-19, Streptococcus pneumoniae is spread in droplets when we cough or sneeze. It is responsible for fatal pneumonia, meningitis, sepsis or blood poisoning, as well as inner ear infections that commonly cause deafness in Aboriginal and Torres Strait Islander children.
Before the COVID-19 pandemic, S. pneumoniae was the leading cause of pneumonia death worldwide. It caused more deaths than all other causes of pneumonia combined in 2016.
This despite the widespread use of antibacterial agents, vaccines and antibiotics to protect against S. pneumoniae infections. Our current vaccines offer limited protection, acting against only a fraction of all strains of S. pneumoniae. The other challenge is that resistance to antibiotics is growing rapidly.
New approaches are urgently needed to prevent death and treat disease and disability caused by S. pneumoniae, such as brain damage, hearing loss and impaired lung function in adulthood.
A new approach may be to starve the pathogen.
“Vitamins and minerals, including iron, copper, zinc and manganese, are essential for human health, but bacteria also need them,” says associate professor Megan Maher, structural biologist and chemist at the School of Chemistry and the University of Melbourne’s Bio21 Institute.
Over a decade ago, Professor Christopher McDevitt, Laboratory Manager at the Doherty Institute, and Associate Professor Maher forged a collaboration to uncover a clue that manganese was vital for the survival, metabolism and infectivity of S. pneumoniae.
Professor McDevitt, a microbiologist, first identified that zinc was toxic to S. pneumoniae because it prevented manganese from entering the cell.

The protein crystals are grown in very small drops (about 1 microliter) from pure preparations so that their structure can be determined by X-ray crystallography. Credit: Megan Maher
“Our work showed that the pathogen had to steal manganese from the body to cause infection. Part of the body’s immune response against the bacteria was to use zinc to block this pathway,” says Professor McDevitt.
Knowing that manganese was an essential mineral for the pathogen, Professor McDevitt and his team researched and identified the “gate” or transporter in S. pneumoniae that is specific to manganese uptake.
“S. pneumoniae has only one transporter to take up this essential nutrient. We already knew that if you disrupted any part of this transporter, the pathogen was unable to cause infection. Since this type of transporter is not in humans, it makes an ideal target for new antimicrobials, ”says Professor McDevitt.
“The ultimate goal was to determine the structure of the transporter used by S. pneumoniae, in order to design drugs against it. At the time, it was very difficult to do, so Professor McDevitt approached me and Mr. ‘asked, shall we go? ” recalls Associate Professor Maher.
“To understand how the transporter works, we used X-ray crystallography,” says Associate Professor Maher.
This technique requires crystals of the protein to be grown in the laboratory, and then an X-ray beam is directed at the crystals. For each crystal, light diffuses in a unique way, known as a diffraction pattern, to build a precise three-dimensional structure of the protein. These 3D structures are represented as beautiful curls, leaves and spirals.
But extracting the manganese transporter from S. pneumoniae was a particularly difficult task because it was tightly integrated into the bacterial cell membrane, where it serves to control the entry of metal into the cell.
Cell membranes act as a barrier between the interior of a cell, which contains the machinery necessary for life, and the outside world. It is made up of fatty or “lipid” molecules. This makes the membrane proteins located within them particularly difficult to isolate.
“It took us six years, including time spent at the University of La Trobe, and was completed at the Bio21 Institute. Membrane proteins are among the most difficult classes of proteins you can work on and not therefore represent only a tiny proportion of all known protein structures because they are so difficult, which is why it took six years, ”explains Associate Professor Maher.
“We used detergents to remove the protein from the membrane and keep it soluble in solution. Once the protein was enclosed in a shield of detergent molecules, we grew crystals with the detergent present.”
The protein crystals are grown under highly controlled conditions. It can take weeks, months, and many attempts to get the right conditions to grow them.
“This manganese transporter certainly had a lot of surprises in store for us. It belongs to a very large family of proteins called ‘ABC transporters’ but looks quite different from others of the same family, the structures of which have already been revealed”, explains the professor. aggregate. Maher.
“It is smaller and more compact than other ABC transporters and has a distinct pathway through which manganese travels. Similar ABC transporters have been referred to as ‘Teflon-coated channels’ where once the correct molecules are received by the transporter , they just slide through her into the cell without any interaction, ”she said.
“What we discovered is a unique pouch midway through the carrier, which specifically interacts with manganese.”
Membrane proteins that function as gateways in cell membranes are of particular interest as drug targets because of their important role in the transport of essential molecules – like manganese – as well as in signaling and other functions.
“Manganese is a tiny chemical species. In order for the transporter to specifically select it, there has to be an interaction inside the gateway to help get the right molecule through. This is an example of” coordination chemistry. ” “, explains Professor McDevitt.
“With a lot of other soluble proteins, we can predict what they’re going to look like because we already have so many other structures in the databases that we can compare ourselves to. Because we don’t have a lot of structures for them. membrane proteins, when you get a new structure it’s often quite different from the ones that already exist, ”says Professor McDevitt.
“Knowing the structure is the first step in developing a drug or therapy against S. pneumoniae. Our approach would be to block this pathway and prevent the transporter from bringing manganese into the bacteria, ”explains Professor McDevitt.
“This can be done by creating specific antibodies or other binding molecules that specifically target this transporter and block it. That’s what we’ll be working on next,” says associate professor Maher.
Traditionally, antibiotics against S. pneumoniae target cell wall production, but currently there are no antibiotics that target the body’s nutrition.
Targeting the gateway that allows S. pneumoniae to obtain the essential element manganese is a completely new approach and a positive step towards a new way to tackle the increasing rate of antibiotic resistance.
Starving Pneumonia-Causing Bacteria From Favorite ‘Food’ Shows Promise For New Antibiotics
The structural basis for importing bacterial manganese, Scientists progress (2021). DOI: 10.1126 / sciadv.abg3980
Provided by the University of Melbourne
Quote: Starving the bacterium that causes pneumonia (2021, August 9) retrieved August 9, 2021 from https://medicalxpress.com/news/2021-08-starving-bacterium-pneumonia.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link