
[ad_1]
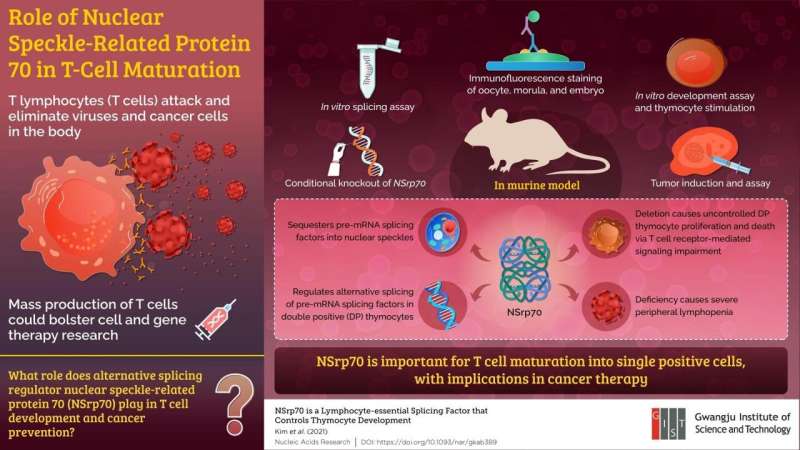
Importance of a protein called NSrp70, which was previously discovered in the subnuclear spaces of T cells, in the regulation of T cell maturation. Credit: Gwangju Institute of Science and Technology
T lymphocytes, or T cells, are immune cells that play various roles in building the body’s immunity. How does a particular cell type fight against a multitude of different pathogens? The key to this adaptability lies in alternative splicing, in which the cell produces multiple forms of proteins to identify different types of invading viruses and microbes, as well as to destroy cancer cells. Not surprisingly, therefore, finding ways to enhance T cell production with improved pathogen recognition ability is an actively researched area of modern science.
In 2011, scientists at the Gwangju Institute of Science and Technology (GIST) in Korea discovered a protein called NSrp70, which is abundant in mobile T cells. Located in specific regions inside the nucleus of the cell called nuclear speckles, NSrp70, which is the abbreviation for nuclear speckle-bound 70 protein, is a gene regulator. Interestingly, nuclear spots play an important role in protein production by harboring mRNA splice regulators that cut and join pre-messenger RNA fragments to produce the final messenger RNAs based on the proteins produced. Armed with this knowledge, scientists speculated on the possible role of NSrp70 in alternative splicing involved in T cell maturation and development.
Now, in a new study uploaded on May 25, 2021 and published in volume 49 issue 10 of the journal Nucleic Acid Research, scientists from GIST, led by Professor Chang-Duk Jun, report their findings on the role of this protein and its encoding gene in a physiological environment. Explaining their motivation for the research, Prof Jun says, “Since we discovered and introduced NSrp70 to academia, we have explored its functions and mechanisms of action.
In experiments with mice, the scientists were able to analyze the protein’s function using a technique known as conditional gene knockdown, whereby they knocked out the gene encoding NSrp70 and produced cell samples lacking the protein. By comparing these cell samples with ordinary cells, they determined the effects caused by the absence of the protein.
They report that NSrp70 is expressed very early in the cell cycle and plays an important role in the development of T cells. Specifically, the absence of the protein induced uncontrolled cell growth and death in doubly positive thymocytes which are T cell precursors. This hampered their progression to single positive thymocytes, preventing the formation of mature T cells. Mice deficient in NSrp70 had significantly reduced lymphocyte counts in peripheral tissues and, as a result, resulted in uncontrolled tumor growth, further attesting to the role NSrp70 plays in cancer development. Prof Jun categorically sums up the results: “Our study found that NSrp70 is an important regulator of T cell proliferation. This finding may help us mass produce specific T cells for cell therapy or use produced T cells. en masse to inhibit the proliferation of cancer cells by gene therapy.
The world eagerly welcomes these critical findings on nuclear spots and constituent proteins.
“Nuclear Speckle” Structures Inside Cells Improve Gene Activity and May Help Block Cancers
Chang-Hyun Kim et al, NSrp70 is an essential lymphocyte splicing factor that controls thymocyte development, Nucleic Acid Research (2021). DOI: 10.1093 / nar / gkab389
Provided by GIST (Gwangju Institute of Science and Technology)
Quote: Subnuclear protein NSrp70 has been shown to be an important regulator of T cell proliferation (2021, July 14) retrieved July 14, 2021 from https://phys.org/news/2021-07-subnuclear-protein -nsrp70-important-cell.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link