.jpg)
[ad_1]
In a recently published study on the medRxiv* preprint server, scientists from Singapore assessed the levels of anti-coronavirus 2 (SARS-CoV-2) specific anti-severe acute respiratory syndrome IgA antibodies in breast milk of women recently immunized with the second dose of mRNA-based coronavirus disease 2019 Vaccine (COVID-19). The results show a lower titer of antibodies against the variants of concern of SARS-CoV-2 (VOCs) in breast milk after vaccination.
.jpg)
Background
Studies conducted in real situations have shown that the COVID-19 vaccine developed by Pfizer / BioNTech BNT162b2 can induce anti-SARS-CoV-2 antibodies in human breast milk. However, it remains unclear whether these antibodies can protect against emerging SARS-CoV-2 VOCs and how long the protection persists. Since clinical studies testing the efficacy of the vaccine have not involved pregnant or breastfeeding women, more concrete studies are needed to investigate the extent of passive immunity in infants.
In the present study, scientists investigated whether BNT162b2 vaccination can induce IgA-specific antibodies in breast milk against the peak receptor binding domains (RBD) of SARS-CoV-2 VOCs. Specifically, they targeted four VOCs, including alpha (first detected in the UK), beta (first detected in South Africa), gamma (first detected in Brazil) and delta variants. (first detected in India).
Study design
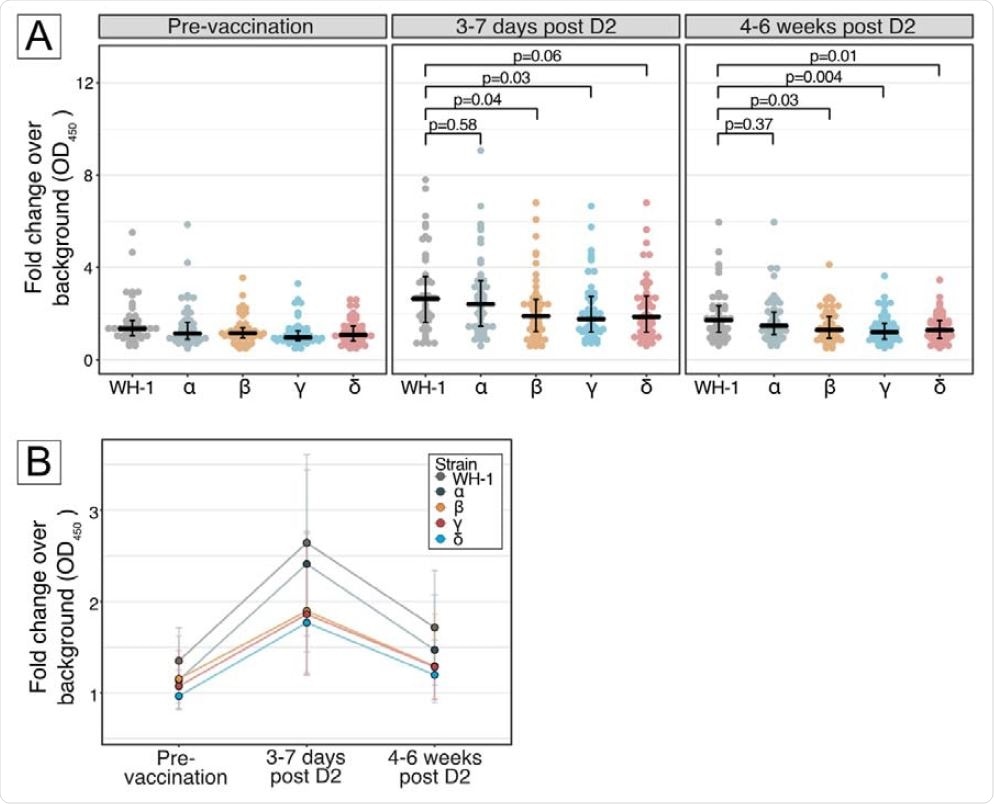
The study was conducted in 46 breastfeeding women who received two doses of Pfizer / BioNTech BNT162b2 vaccine 21 days apart. Milk samples were taken before vaccination (baseline), 3 to 7 days after the second vaccination and 4 to 6 weeks after the second vaccination. The levels of IgA-specific anti-RBD antibodies against the original strain of SARS-CoV-2 and four VOCs were measured using enzyme-linked immunosorbent assay (ELISA).
Important Notes
The results of the study revealed that the antibodies could be detected in breast milk within 3 to 7 days of the second vaccination. In addition, the levels of antibodies developed against the beta, gamma and delta variants were 28-33% lower than those developed against the original viral strain. However, no difference in antibody level was observed between the original SARS-CoV-2 and the alpha variant.
A reduction in antibody levels in breast milk has been observed 4 to 6 weeks after the second vaccination. However, the levels were still higher than the levels before vaccination. Compared to the original SARS-CoV-2, a 25-30% reduction in antibody levels was observed for all VOCs tested except the alpha variant.
Importance of the study
The study reveals that the IgA antibodies induced by BNT162b2 against SARS-CoV-2 and its variants remain detectable in breast milk six weeks after the second vaccination. This indicates that mucosal immunity against SARS-CoV-2 can be transferred from nursing mothers to infants who are not eligible for vaccination and are at high risk for severe COVID-19.
However, the reduction in antibody levels over time observed in the study indicates a rapid decline in vaccine-induced immunity in infants.
As mentioned by scientists, the results of the study are limited to binding antibodies only. No experiments have been conducted to assess the virus neutralizing ability of antibodies. However, there is some evidence to suggest that IgA binding antibodies in the breast milk of vaccinated women positively correlate with neutralization.
Overall, the study highlights the importance of immunizing pregnant women to ensure the transfer of long-lasting passive immunity to infants via the placenta. In addition, breastfeeding mothers should be given priority for vaccination in order to improve the level of protection in susceptible infants.
*Important Notice
medRxiv publishes preliminary scientific reports which are not peer reviewed and, therefore, should not be considered conclusive, guide clinical practice / health-related behavior, or treated as established information.
Source link