[ad_1]
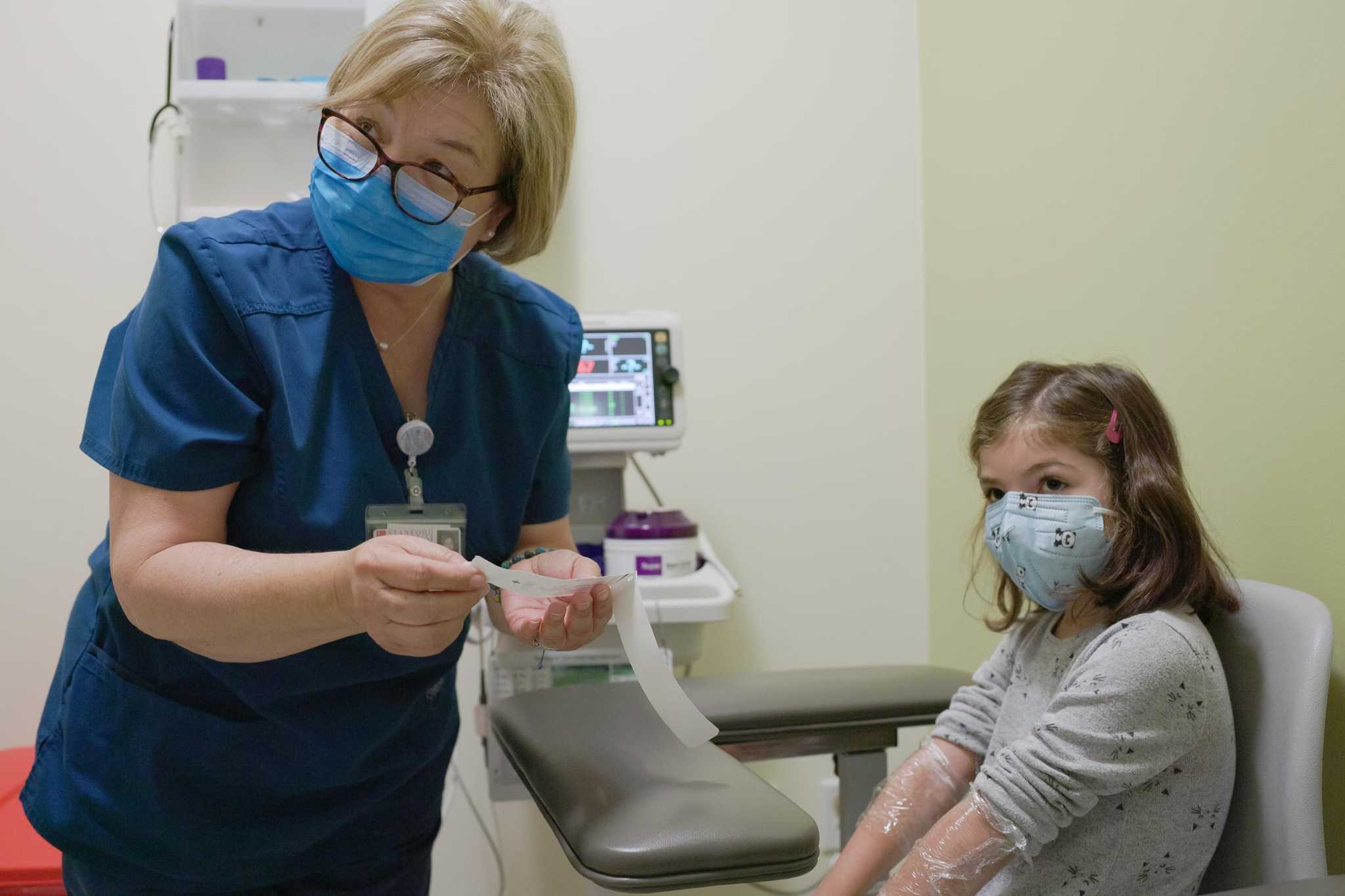
The Pfizer-BioNTech coronavirus vaccine has been shown to be safe and very effective in young children aged 5 to 11, the companies said on Monday. The news paves the way for the vaccine to be approved for young children, possibly before the end of October.
Renee and Miguel Chavez, who enrolled their children in the Pfizer vaccine trial for school-aged children at Stanford, said the news was a “huge relief.”
“Like any parent, we want to do everything possible to protect our children,” said Renee Chavez, who works as a pediatric intensive care nurse at Lucile Packard Children’s Hospital. “I know the masks work. However, vaccines are the gold standard for protecting people. Now, it’s great that the data is there.
Children now account for more than one in five new COVID-19 cases nationwide, and the highly contagious delta variant has sent more children to hospitals and intensive care units in recent weeks than it did. at any other time during the pandemic.
Pfizer and BioNTech plan to apply to the Food and Drug Administration by the end of the month for permission to use the vaccine in these children. If the regulatory exam goes as well as it does for older children and adults, millions of elementary school students could be vaccinated before Halloween.
Trial results for children under 5 are not expected until the fourth quarter of this year at the earliest, according to Dr Bill Gruber, senior vice president at Pfizer and pediatrician.
“The best thing to come for the approval of the vaccine for children will be to relieve the parents of worry,” said Dr. Jenna Bollyky, one of the Stanford Medicine researchers involved in the clinical trials of the vaccine. “Parents and children have been under a lot of stress since returning to school. It will give them a feeling of peace.
There were 225,978 cases of COVID-19 children reported in the United States for the week ending September 16, according to data released Monday by the American Academy of Pediatricians. A small percentage of children who contract the virus have persistent symptoms or an inflammatory syndrome that can affect multiple body systems.
The Bay Area, where vaccination rates are relatively high and universal masking is required except in Solano County, has seen few pediatric hospitalizations, but experts remain vigilant as school continues.
“I tell people if you could see what I see – if you could see what the people who work on the frontlines see every day – you would stop buying conspiracy theories and fighting against vaccines and warrants. every step of the way, ”said Renee Chavez, the pediatric intensive care nurse. “It’s one thing to tell someone that a 3 year old is going to be intubated and go for ECMO. It’s completely different to know what that means.
Children are as likely as adults to pass the virus to others, and more likely than adults over 60, according to a recent review of the evidence by the Centers for Disease Control and Prevention.
“It’s always a priority for them,” said Miguel Chavez of his 9-year-old son, Nico, and 6-year-old daughter, Sofia, who were eager to get the vaccine to not only protect themselves, but also extend family members. “They knew the vaccine was the light at the end of the tunnel, and they definitely felt left out. “
Pfizer’s trial included 2,268 children aged 5 to 11, two-thirds of whom received two doses of the vaccine three weeks apart; the others were injected with two doses of saltwater placebo. The results are not yet peer reviewed.
The Chavez were not told whether their children received the vaccine or the placebo; they suspect it was the vaccine.
Children in the 5 to 11 age group had a strong antibody response with 10 micrograms of the vaccine, one third of the dose given to older children and adults.
At higher doses, researchers observed more side effects in young children, including fever, headache and fatigue, although none were serious, Gruber said. With the 10 microgram dose, “we actually see after the second dose, less fever, less chills than in 16 to 25 year olds. “
The immune system weakens with age and the side effects also subside. This drop in potency is why most vaccines are given in childhood – and why a much lower dose is often sufficient for children, said Dr Yvonne Maldonado, who led the trial at Stanford and chairs the American Academy of Pediatrics Infectious Disease Committee. .
“You want to reach the sweet spot, where you are giving the lowest dose that could cause reactions, but also high enough to get you a good, long-lasting antibody response,” she said.
In children under 5, only 3 micrograms – a tenth of the adult dose – are tested in trials and appear likely to be sufficient, she said.
“The science that was used to make these vaccines was based on so much research. What a powerful thing for us to witness, that the vaccine can come out so early and protect us, ”said Renee Chavez, who added that her family will contact the study team every week for the next two years with reports. written. , meetings organized on Zoom and regular phone calls. “When they say it’s safe and effective, it’s the amount of research that goes into it. “
In order to detect side effects in young children, the FDA in July asked Pfizer-BioNTech and Moderna to expand their trials to 3,000 children. But based on the company’s conversations with the FDA, Gruber said he believed the agency would give the vaccine the green light with the data available.
Chronicle editor Aidin Vaziri contributed to this report.
Apoorva Mandavilli is a New York Times writer.
[ad_2]
Source link