[ad_1]
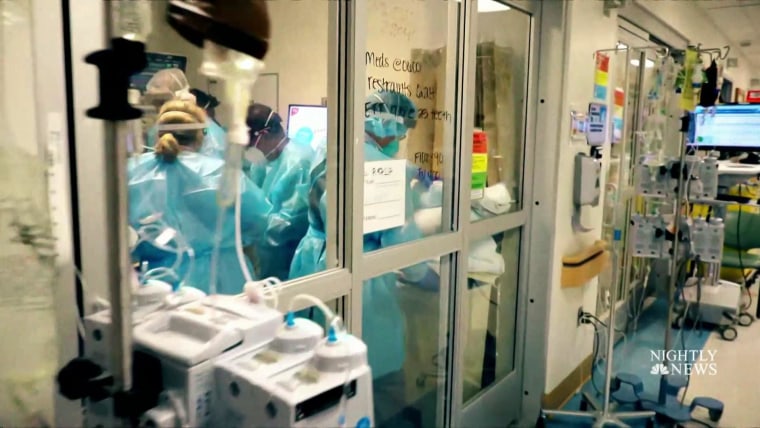
A drug that could protect high-risk Covid-19 patients from developing serious illness sits on unused shelves as record numbers of people are hospitalized in the United States.
State and federal public health officials on Thursday are advocating with the country to take advantage of its vast offering of monoclonal antibody treatments, the only therapy available that can potentially keep patients out of hospital.
Comprehensive coverage of the coronavirus outbreak
“This is the first time during the pandemic that I can remember that our resources have far exceeded demand,” Dr William Fales, medical director of the Michigan Department of Health and Human Services, said Thursday at a press briefing organized by the US Department of Health. Health and social services. Fales estimated that only 10% of Covid-19 patients in the state who are eligible for therapy had received it.
Monoclonal antibodies are drugs made in the laboratory to mimic natural antibodies against SARS-CoV-2, the virus that causes Covid-19. They are recommended for people at high risk of contracting the virus, including people over 65 and those with underlying health conditions.
At least one study has shown that therapy can reduce the amount of virus in a person’s system. But no benchmark research proves that monoclonal antibodies actually provide this benefit. Most of the reports are anecdotal.
Fales said his team had observed that hospitalization rates in the two weeks following monoclonal antibody treatment appeared to be around 5%. That’s about half the rate of patients who received placebos in monoclonal antibody therapy studies from drug maker Regeneron, according to the drug’s emergency clearance by the Food and Drug Administration.
Dr Andrew Thomas, clinical director of Wexner Medical Center at Ohio State University, suggested during a media call on Wednesday that the use of monoclonal antibodies had relieved strain on the hospital system.
Thomas said his system “sped up” the use of monoclonal antibodies quickly. “I would like to think that this is the reason why our hospitalizations have decreased,” he said.
Dr Jonathan Parsons, head of the monoclonal antibody treatment efforts at central Ohio, said, “Anyone who is tested through our swab program is listed in an electronic medical record. Parsons staff then contact primary care providers for patients who test positive, asking if they would like to refer patients for monoclonal antibodies.
New Jersey state epidemiologist Dr Eddy Bresnitz said monoclonal antibodies may have played a role in the recent leveling of Covid-19 hospitalizations in the state. “It’s worth getting it,” Bresnitz said at a press briefing Thursday.
So why don’t people get it?
Simply put, a lack of time, resources and awareness.
Obstacles to administration
Monoclonal antibodies should be given soon after a person has tested positive. “These drugs work best when given early,” Surgeon General Jerome Adams said in Thursday’s briefing.
The two monoclonal antibody products that have been cleared for emergency use by the FDA, from drug makers Eli Lilly and Regeneron, must be administered within the first week of illness.
But with testing still lagging behind in much of the country, many patients have to wait several days to find out if, in fact, they have been infected. Simply waiting for test results can push patients past when they might be eligible for treatment.
This obstacle, however, should not be a factor in obtaining monoclonal antibodies, said Dr John Redd, chief medical officer in the office of the assistant secretary of health and human services for preparedness and response.
“Getting these therapies doesn’t require a PCR test,” Redd said in Thursday’s briefing. (A PCR, or polymerase chain reaction, test is considered the gold standard, but it can take days to get a result.)
Instead, Redd said, “a quick test is quite appropriate.” Rapid tests can return results within minutes, but they have higher false negative rates.
Those on the front lines of treatment for Covid-19 patients say it’s not that easy.
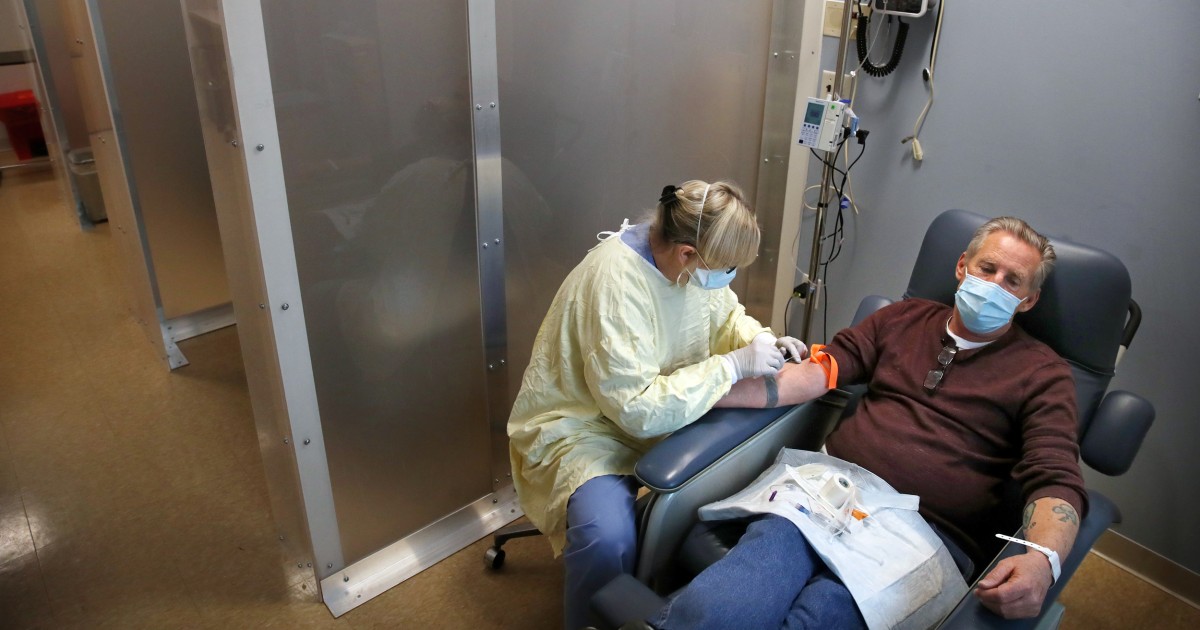
Monoclonal antibodies are given intravenously, as a one hour infusion, with a three to four hour appointment. Because Covid-19 patients are contagious, they must be separated from other vulnerable patients who need outpatient infusions, such as those receiving chemotherapy for cancer.
Dr Peter Chin-Hong, an infectious disease specialist at the University of California, San Francisco, said some patients may decline treatment simply because they feel better. But that could be a mistake. It has become clear that some patients can feel better before they suddenly get worse.
For many others, logistical challenges arise.
Public transport and carpooling, such as Uber, are out of the question for those with active Covid-19. Also, Chin-Hong said, some patients simply cannot afford three hours of time off from work or family obligations.
Chin-Hong estimates that his healthcare system has used less than 20% of the monoclonal antibodies in stock.
In addition, special infusion centers should be set up and staffed. Some say this is an unreasonable demand on health systems that are already overburdened.
“If we had this pandemic under control, we could set up infusion centers. We could set up rapid tests. But we don’t have these resources,” said Dr Pieter Cohen, who is an associate professor at the Harvard Medical School doctor at the Cambridge Health Alliance Respiratory Clinic near Boston.
“We are completely overwhelmed with sick patients,” Cohen said.
Chin-Hong agreed. “These patients are generally fine and you want to focus on the sick patients,” he said.
“I think that’s where the mindset is – especially in California right now,” he said. The state has seen an upsurge in Covid-19 cases in recent times. In the state’s most populous county, Los Angeles, an average of 10 people test positive for the virus every minute.
The hurdles are not lost for at least some of those leading the federal response. “We recognize that the health care system is very stressed,” said Dr. Janet Woodcock, Therapeutics Manager for Operation Warp Speed, during Thursday’s media call.
“On the flip side, if we don’t, it’s likely we’ll have even more overwhelmed hospitals and healthcare workers,” Woodcock said, adding that his team believes efforts to put in place such infusion centers are “worth it” to reduce the burdens on health systems.
Some stand-alone renal dialysis centers across the country have announced that they will begin administering monoclonal antibodies to Covid-19 patients during shifts set up for these patients only. Covid-19 has been shown to be particularly serious for patients with kidney disease.
Download the NBC News app for comprehensive coverage of the coronavirus outbreak
Another factor may be the lack of awareness, both among patients and providers, that treatments are available.
In a press conference Tuesday, Health and Human Services Secretary Alex Azar put the burden of the prosecution of monoclonal antibodies on patients, who “should ask their doctors or health care providers why they are not offered these antibody therapies “.
However, HHS ‘online tool provides little help to those trying to find monoclonal antibody resources. The site does not contain data on people from at least 31 states, including Alabama, Kansas, Michigan, New Jersey, New York, North Carolina and Washington.
A spokesperson for HHS said Thursday the team is working “as quickly as possible” to update the site and expects more resources to be available by next week.
Follow NBC HEALTH on Twitter & Facebook.
[ad_2]
Source link