
[ad_1]
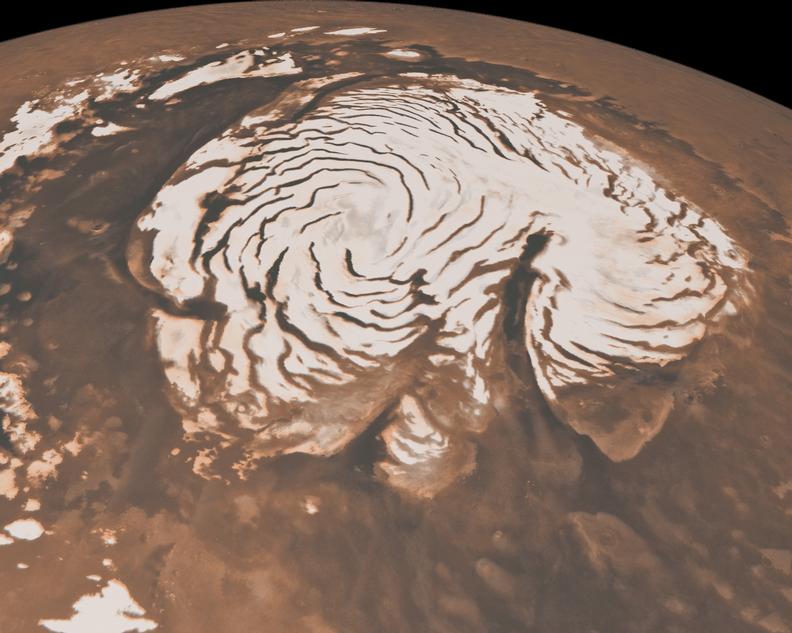
Mars clearly had a hot, humid past, a time when streams, lakes, and even an ocean were present on its surface. Currently, however, most of the planet's water seems to be locked in its icy poles, and the atmosphere is so thin that water would evaporate quickly even if temperatures were maintained at levels similar to those of the Earth. But could we return to the future? Is there enough material on Mars to form a dense atmosphere filled with greenhouse gases sufficient to keep the water hot?
Elon Musk, CEO of SpaceX, suggested that we could do this simply by atomizing Mars. poles, releasing ice (water and ices of carbon dioxide) into the atmosphere. Asked about the plan's prospects, a scientist said, "That it really works, I do not think anyone has worked on physics in any detail to say that would be the case." Now, two planetary scientists have accepted the challenge of working on physics, and they have bad news for Elon.
Greenhouse and pressure
Researchers Bruce Jakosky and Christopher Edwards focus on two important issues. The first is whether we can put enough gas back into the atmosphere to create atmospheric pressure similar to that of the Earth, so that people who have to do something on the surface do not need to wear bulky combinations to isolate from the environment. The second is to know if we can sufficiently heat the surface so that the liquid water can persist there.
One or the other of these substances requires a lot more gas in the atmosphere. This is because the gases, in addition to increasing the pressure, can mediate the greenhouse effect, capturing the outgoing radiation before it reaches the space and converting a portion into kinetic energy. As it happens on Earth, it has the net effect of raising temperatures. I'm releasing enough good gas, you can handle both of these problems.
The trick is to find the right gas. Water vapor is a potent greenhouse gas, but it is difficult to get much of it into a cold atmosphere before it starts to precipitate; it improves the warming caused by other factors, but can not drive it alone. People have also suggested pumping in CFCs, which are also potent greenhouse gases, but these are not chemically stable, so they could not lead to long-term warming. All this takes us back to carbon dioxide, a greenhouse gas that is already 90% of the Martian atmosphere.
Unfortunately, this atmosphere is only enough to generate about 0.6% of the pressure of the Earth's atmosphere. And this is clearly not enough to keep the planet very hot. In fact, it is so cold that part of the atmosphere freezes at one of the poles during the seasonal cycle of Mars.
After considering the options, the researchers' challenge is simplified: can you identify enough CO sources? 2 on Mars to fill its atmosphere?
Inventory
Regarding this seasonal ice, researchers estimate that it accounts for about one – third of what is already in the atmosphere. The radar imaging of polar ice caps indicates that there are also layers of carbon dioxide ice. Assuming that this can be put back into the atmosphere, and you would double the current atmospheric concentrations. Together, this brings us to about 1.5% of Earth's atmospheric pressure.
From there, however, things become very difficult and speculative. For example, water ice may form in such a way that it encapsulates individual gas molecules in its crystalline structure (on Earth, it is usually methane / ice mixtures). These "clathrates" may contain carbon dioxide on Mars, but it is impossible to say how much ice (if there is any) is in this form. But even if every piece of ice on the planet is a clathrate of carbon dioxide, just reach the atmosphere at 15% of Earth's pressure, assuming we were ready to bomb the poles and all glaciers to melt the ice. [19659003] Other pieces of carbon dioxide from Mars are associated with its rocks. Some of them are vaguely attacked on the rocky surfaces; this is especially true for loose and sandy materials, which have more surface area to adsorb gas. This can be warmed up, but it faces a number of problems. For starters, even though we have taken a high estimate for CO 2 locked up this way, this only pushes atmospheric levels up to about half of what we want. And in doing so, it would have to heat up pretty much everywhere on the planet, where there is rock – think of an open pit mine on the Mars scale. Finally, the carbon dioxide retained by the rocks is in equilibrium with that of the atmosphere. It would begin to adsorb as soon as we stop heating the rock.
The last possibility seen by Jakosky and Edwards is that of carbonate minerals. These can undergo chemical transformations that release carbon dioxide, leaving a different mineral behind. But we have imagined a large part of the Martian surface, and there is simply not much carbonate there. There is a region, called Nili Fossae, where they are visible. If researchers assume that what we see on the surface is a sign of larger deposits below them, then there is a significant amount of carbon locked up there. If we destroy the region and release into the atmosphere, current levels would probably be tripled. With maximum optimism, it could give us 15% of the atmospheric pressure of the Earth.
If you combine the most optimistic estimates, you will get close to 80% of the Earth's atmospheric pressure. But that would require us to melt the two poles, strip the entire surface of the planet and heat each piece of rock – and heat it indefinitely. More realistic, Jakosky and Edwards think that we could probably triple the current levels of carbon dioxide in the Martian atmosphere, which would only bring us 1.5% of the pressures we see on Earth. If we generously give a figure of 2%, then we are looking at 10K of additional greenhouse warming, which is far from allowing liquid water to surface.
Lost in Space
So how did Mars ever end up with streams and lakes? Conveniently, we have studied this question, using instruments on orbits such as MAVEN and Mars Express. These followed the continued loss of gases from the Mars atmosphere, which are lost in space as the solar wind and other radiations slam into the planet. Using numbers on the loss of oxygen from these observations, and by tracking the amount of radiation produced during the history of Sun-like stars, it is possible to estimate how many Oxygen has been lost on the history of Mars. lost to increase the atmospheric pressure of Mars to levels similar to the Earth, or to cover its surface in a layer of water several tens of meters deep. Estimates based on isotopic carbon ratios (light isotopes are lost in space more easily) indicate that Mars has also lost half of its initial carbon. "The loss of space was the dominant process to suppress the old greenhouse atmosphere," conclude Jakosky and Edwards
And, unfortunately, once something is lost in space, he does not return. So, realistically, to terraform Mars, we seek to obtain this material from a non-local source. Of course, breaking comets and asteroids in the poles would also melt the ice caps, so it's a little two to one. But it's far beyond anything even Elon Musk considers in his states of optimism.
Nature Astronomy 2018. DOI: 10.1038 / s41550-018-0529-6 (About DOIs).
Source link