
[ad_1]

The terms "misleading" and "false" are two words used by the Federal Trade Commission (FTC) in the United States to describe the marketing claims of two stem cell clinics in California.
According to a FTC complaint, clinics had announced that they were using amniotic stem cell therapy to successfully treat serious conditions such as multiple sclerosis, autism, stroke and seizures. heart. The claims included an announcement that their treatment had restored the sight of a 101-year-old woman who had been blind for seven years.
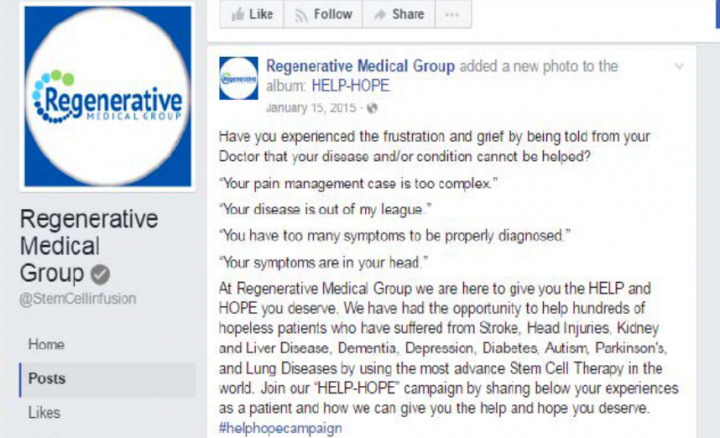
A promotional letter included in the complaint states: "Lives are saved, the blind see, crippled walking and patients with heart, lung, kidney and nerve diseases can change the course of their suffering through therapy. simple. … "
There is no evidence that it works
According to the FTC, clinics treated patients with stem cells derived from the amniotic fluid of cesarean women. (Amniotic stem cell therapy should not be confused with autologous stem cell transplants, in which the stem cells are extracted from the patient's body and replaced after chemotherapy.)
In his complaint, the FTC stated that there was no evidence that any of the treatments provided by the clinics was working. "The statements … are false or were not justified at the time the representations were made. In fact, many of these diseases are currently considered incurable by health professionals, "said the agency.
FTC demands a return on investment
A press release from the FTC said:
"Dr. Bryn Jarald Henderson, DO and the two companies he owns and operates, Regenerative Medical Group and Telehealth Medical Group, have earned at least $ 3.31 million by offering stem cell therapy between 2014 and 2017. The injections Initial stem cell treatments ranged from $ 9,500 to $ 15,000, with patients being encouraged to take multiple treatments. Follow-up "reminder" treatments cost between $ 5,000 and $ 8,000 each.
In the proposed Regulations, Dr. Henderson and his companies are prohibited from making health claims "unless these claims are true and supported by competent and reliable scientific evidence". The regulation requires companies to pay the FTC a partially suspended fine of $ 3.31 million, the approximate amount the commission paid to the company between 2014 and 2017. Of this amount, $ 525,000 would be reserved for patients who may have been harmed by the treatments.
The moral of this column, of course, is patient, beware. If a treatment seems too good to be true with unverified affirmations of success, it's probably wise to stay away.
You are invited to follow my personal blog on www.themswire.com..
***
Note: News about multiple sclerosis today is strictly a site of information and information about the disease. It does not provide any medical advice, diagnosis or treatment. This content is not intended to replace the advice, diagnoses or treatments of a health professional. Always seek the advice of your doctor or other qualified health care professional about any health problem. Never neglect professional medical advice or be quick to find it because of something you read on this site. The opinions expressed in this column are not those of News about multiple sclerosis today or its parent company, BioNews Services, and is intended to spark discussion on issues related to multiple sclerosis.
[ad_2]
Source link