[ad_1]
The report analyzed positive overdose deaths for fentanyl and similar compounds from July 2016 to June 2017 in 10 states: Kentucky, Maine, Massachusetts, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia and Wisconsin. From July 2016 to December 2016, the CDC found 764 fentanyl- and fentanyl-related deaths in the 10 US states. Over the next six months, from January 2017 to June 2017, the CDC reported 1,511 overdose deaths involving drugs.
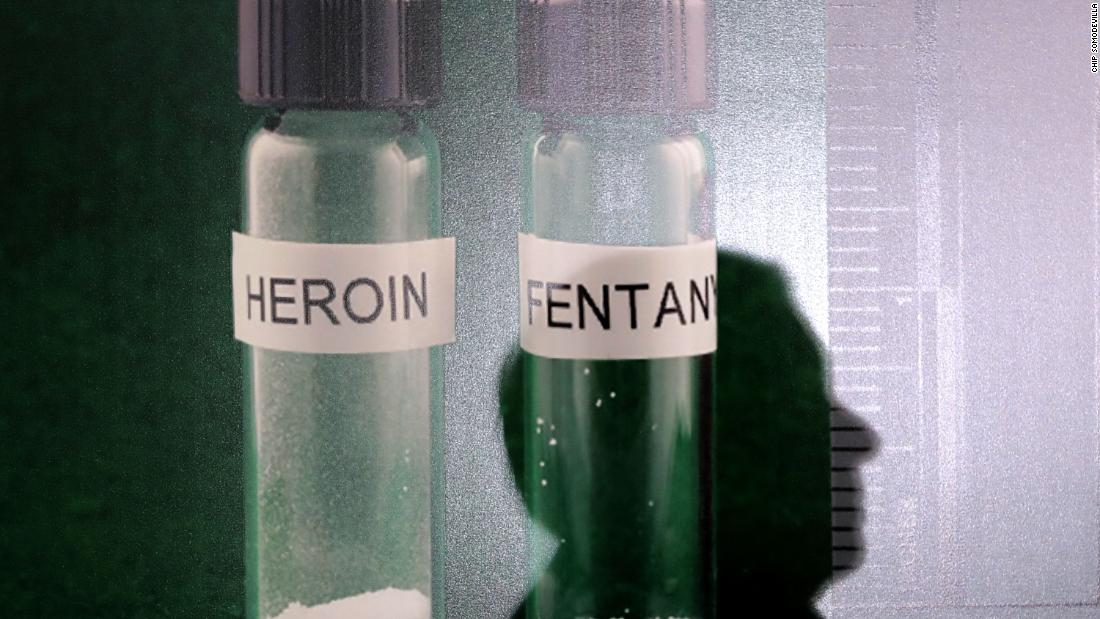
When it is legitimately prescribed, fentanyl helps patients manage extreme pain, such as that caused by cancer. It is usually dosed as tablets, patches or intravenously. However, the illicit forms of the drug are usually sold in powder form or pressed into pills. Fentanyl and chemically similar variations, known as analogues have been sold on the black market and can be extremely potent.
Such an analogue is the drug carfentanil. It is 10,000 times more powerful than morphine and used to calm elephants. The drug was responsible for overdose outbreaks in Ohio and West Virginia in 2016. The CDC found that deaths associated with carfentanil jumped 94% from 421 to 815 in the 10 states studied during of the 12-month period.
Nationally, figures for 2017 are still preliminary, but the CDC expects opioid overdose deaths to reach an all-time high of 49,000. 60% of these victims should be related to synthetic opioids such as fentanyl and carfentanil. According to the CDC, there were 20,310 overdose deaths involving synthetic opioids in 2016, and this number is expected to increase by 45% to 29,400 in 2017.
The growing popularity of these synthetic products has been called the third wave of the epidemic. # 39; opioids; the first wave was attributed to overescription of painkillers like oxycodone and hydrocodone and the second to heroin. The drugs are all chemically similar and act on the same receptors in the brain.
This increase in overdoses related to illicit fentanyl and its analogs reflects an increase in drugs submitted to the Drug Enforcement Administration that tested positive for fentanyl. According to the DEA, the fentanyl positive samples tested by the agency increased from 14,400 in 2015 to 34,119 in 2016. In the first six months of 2017, the DEA is expecting that 25,460 submissions were tested positive for the drug.
CDC issues alert
This report is a follow-up to a CDC alert this week to public health professionals, first responders and medical examiners about the increased availability of synthetic opioids unlawful. The alert is an update of a health advisory from October 2015.
The updated notice warned that fentanyl was detected in association with drugs such as benzodiazepines, counterfeit opioids, ketamine and methamphetamine.
The warning warned health care providers that multiple doses of naloxone, the antidote to opioids, may be needed to revive some of an overdose.
Operation SOS
In an attempt to stem the tide of overdoses related to illicit fentanyl, US Attorney General Jeff Sessions announced a campaign on Thursday. aggressive Ministry of Justice. distribution of fentanyl, fentanyl analogues and other synthetic opioids, regardless of the amount of drug, in the 10 districts with the highest rates of overdose in the country. The Operation Synthetic Opioid Surge is focused on the districts of California, Kentucky, Maine, New Hampshire, Ohio, Pennsylvania, Tennessee and West Virginia. The fentanyl bust in Nebraska records doses sufficient to kill about 26 million people. 19659018] The record bust of fentanyl in Nebraska finds enough doses to kill about 26 million people "class =" media_image "src =" http://cdn.cnn.com/cnnnext/dam/assets/160428183940-fentanyl- opioids-drugs-overdose-sanjay-gupta-mobile-orig-mss-00000000-large-169.jpg "
The effort to counter the overdose crisis has reaches out to all corners of public health and government.
Also on Thursday, Democratic Senator Claire McCaskill of Missouri released a report on the practices of drug distributors.The survey revealed that three of the largest distributors of Countries – McKesson, AmerisourceBergen and Cardinal Health – shipped 1.6 billion pills to the state of Missouri between 2012 and 2017. This equates to an average of over 260 tablets per capita of the state during each of these years.
Congressional Survey of Distribution Practices
[19659002] The McCaskill Inquiry focused on Missouri alone but identified failures in the broader pharmaceutical distribution process.
The three distributors had inconsistencies in the reporting of suspicious orders over the past 10 years, the report says. Under the Controlled Substances Act, distributors must monitor and report suspicious orders to the DEA, which requires distributors to report orders "of an unusual size, with orders substantially moving away from orders. a normal pattern and unusual frequency orders 'to agencies' field offices. Distributors are wholesalers who work as intermediaries between manufacturers and hospitals and pharmacies.
The report revealed that the three distributors "systematically failed" when reporting.
For example, McKesson and AmerisourceBergen shipped approximately 650 million tablets to Missouri between 2012 and 2017. During this period, McKesson reported more than 16,700 suspicious orders at the DEA while AmerisourceBergen made 224 reports of suspicious orders during this period. Cardinal Health reported 5,125 questionable orders to the DEA
Although the report states that the results themselves did not find anything illegal, it highlighted potential failures in the system that could lead to the diversion of legal drugs to the black market.
"The opioid crisis that these pills have fueled is a failure of government policy and oversight and a failure of fundamental human morality by many drug and drug companies – a failure that has destroyed Families and communities all over the world, state, "McCaskill said in a statement
Firms Confident in Their Practices
In response to the report, McKesson stated that" we have invested resources important in our anti-diversion program and, since 2008, have blocked and reported more than one million suspicious orders nationwide. "
AmerisourceBergen echoed its commitment to help end to the opioid crisis and to follow suspicious orders. In a statement, the company said, "AmerisourceBergen's order monitoring program has two key steps: locating orders to review with the help of our complex computer algorithm and conducting a detailed investigation of each order. reported. highly accurate and achievable. "
The Healthcare Distribution Alliance, a national trade association representing distributors, criticized the senator's report, saying in a statement that the report is based on" demystified and inaccurate claims without recognizing the need for broader reforms ". in the pharmaceutical supply chain "and points out that doctors are now touting themselves as" main "contributors to the crisis.
Potential drop in opioid dependence rates
Also on Thursday, a Blue Cross Blue Shield report has shown potential progress in efforts to combat the opioid crisis.New figures from the insurance giant have revealed that the number of people with opioid addiction (also known as the name of opioid use disorder) dropped for the first time in the eight years that the company knew ivies.
In 2016, diagnoses of opioid addiction peaked at 6.2 cases per 1,000 members of Blue Cross Blue Shield. In 2017, this rate decreased slightly to 5.9 cases per 1,000 members.
According to the Administration of Addiction and Mental Health Services, about 2 million Americans have a disorder of opioid use. However, only one in five gets specialized treatment. According to Blue Cross Blue Shield, approximately 40% of its clients diagnosed with opioid use disorder have access to specialized treatment.


