
[ad_1]

The team’s experimental water separation device. Credit: Cockrell School of Engineering, University of Texas at Austin
For decades, researchers around the world have searched for ways to use solar energy to generate the key reaction to produce hydrogen as a clean energy source: the splitting of water molecules to form hydrogen and oxygen. However, most of these efforts failed because doing well was too expensive, and trying to do it at low cost led to poor performance.
Now researchers at the University of Texas at Austin have found an inexpensive way to solve half the equation, using sunlight to efficiently separate oxygen molecules from water. The discovery, recently published in Nature Communication, represents a step forward towards greater adoption of hydrogen as a key component of our energy infrastructure.
As early as the 1970s, researchers were studying the possibility of using solar energy to produce hydrogen. But the inability to find materials with the right combination of properties for a device that could effectively carry out key chemical reactions kept it from becoming a mainstream method.
“You need materials that absorb sunlight well and, at the same time, do not degrade while the water separation reactions take place,” said Edward Yu, professor in the Department of Electrical and Computer Engineering. of the Cockrell School. “It turns out that materials that absorb sunlight well tend to be unstable under the conditions required for the water separation reaction, while materials that are stable tend to be poor light absorbers. solar. These conflicting demands lead you to a seemingly inevitable compromise. , but by combining several materials, one which effectively absorbs sunlight, such as silicon, and another which provides good stability, such as silicon dioxide, into one device, this conflict can be solved. “
However, this creates another challenge: the electrons and holes created by the absorption of sunlight in the silicon must be able to move easily through the layer of silicon dioxide. This generally requires that the silicon dioxide layer does not exceed a few nanometers, which reduces its effectiveness in protecting the silicon absorber from degradation.
The key to this breakthrough is a method of creating electrically conductive paths through a thick layer of silicon dioxide that can be made inexpensively and suited to high manufacturing volumes. To achieve this, Yu and his team used a technique first deployed in the manufacture of semiconductor microchips. By covering the silicon dioxide layer with a thin film of aluminum, then heating the entire structure, arrays of nanoscale aluminum “spikes” that completely bridge the silicon dioxide layer. are formed. These can then be easily replaced with nickel or other materials that help catalyze water splitting reactions.
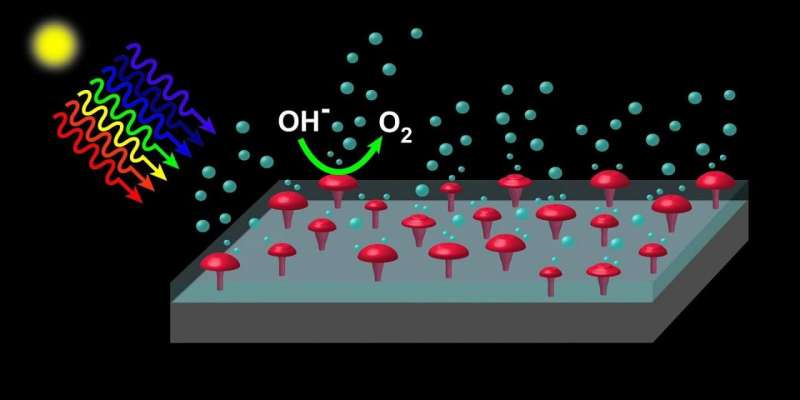
The graphic shows the basic geometry and functionality of the photoanode device. Credit: Cockrell School of Engineering, University of Texas at Austin
When illuminated by sunlight, the devices can effectively oxidize water to form oxygen molecules while also generating hydrogen at a separate electrode and exhibit exceptional stability during prolonged operation. Since the techniques used to create these devices are commonly used in semiconductor electronics manufacturing, they should be easy to scale for mass production.
The team has applied for a provisional patent to commercialize the technology.
Improving the way hydrogen is generated is key to its emergence as a viable fuel source. Most hydrogen production today is done by heating steam and methane, but this relies heavily on fossil fuels and produces carbon emissions.
There is a push towards “green hydrogen” which uses more environmentally friendly methods to produce hydrogen. And simplifying the water separation reaction is a key part of that effort.
Hydrogen has the potential to become an important renewable resource with unique qualities. It already has a major role in important industrial processes, and it is starting to appear in the automotive industry. Fuel cells show promise for long-haul trucking, and hydrogen technology could be a boon for energy storage, with the ability to store excess wind and solar power produced when conditions are right. for them.
In the future, the team will strive to improve the efficiency of the oxygen part of the water separation by increasing the reaction rate. The next big challenge for researchers then is to move on to the other half of the equation.
“We were able to deal with the oxygen side of the reaction first, which is the hardest part,” said Yu, “but you have to do both the hydrogen and the oxygen evolution reactions. to completely separate the water molecules, which is why our next step is to seek to apply these ideas to make devices for the hydrogen part of the reaction. ”
New promising porous material for making renewable energy from water
Soonil Lee et al, Scalable and Highly Stable Metal-Insulator-Semiconductor-Based Photoanodes for Water Oxidation Made Using Thin Film Reactions and Electroplating, Nature Communication (2021). DOI: 10.1038 / s41467-021-24229-y
Provided by the University of Texas at Austin
Quote: Producing clean hydrogen is difficult, but researchers have just solved a major obstacle (2021, July 19) recovered on July 19, 2021 from https://phys.org/news/2021-07-hydrogen-hard-major -hurdle.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link