[ad_1]
Dubai, United Arab Emirates (CNN) – Miranda Kelly, a 44-year-old certified nursing assistant, feared the severe “Covid-19” symptoms afflicting her, including shortness of breath, on the first day of the post of his positive test result. These symptoms were enough to send her to the emergency room, especially since she suffers from high blood pressure and diabetes.
When her husband, Joe, 46, contracted the coronavirus, she worried about her five teenage children. She said, “I prayed that we didn’t need respirators. We have children. Who will take care of them?
After their diagnosis, Miranda and Joe agreed to participate in a clinical trial at the American Cancer Research Center “Fred Hutch”, which is part of an international effort to choose an anti-coronavirus drug that ends her symptoms. .
The next day, the couple took four tablets of the drug, twice a day. They didn’t know if they had taken the real medicine or the placebo. But their symptoms eased within a week, and they recovered after two weeks.
“I don’t know if I was given the test drug, but I’m pretty sure,” Miranda said. “Considering the basic symptoms we were experiencing, I think the recovery process was very quick,” she added.
The Kelly family took part in an experimental step to develop a drug that will likely be the next way out in the fight against the Corona virus, taking anti-drug pills daily after being diagnosed with the virus early, which helps curb the disease. development of Corona. symptoms.
Against this background, Timothy Sheehan, a virologist at the University of North Carolina-Chapel Hill, who pioneered these treatments, explained that oral antiviral drugs have the ability not only to shorten the duration of symptoms. of “Covid-19”. , but also reduce Transmission to people who live with the patient in the same house.
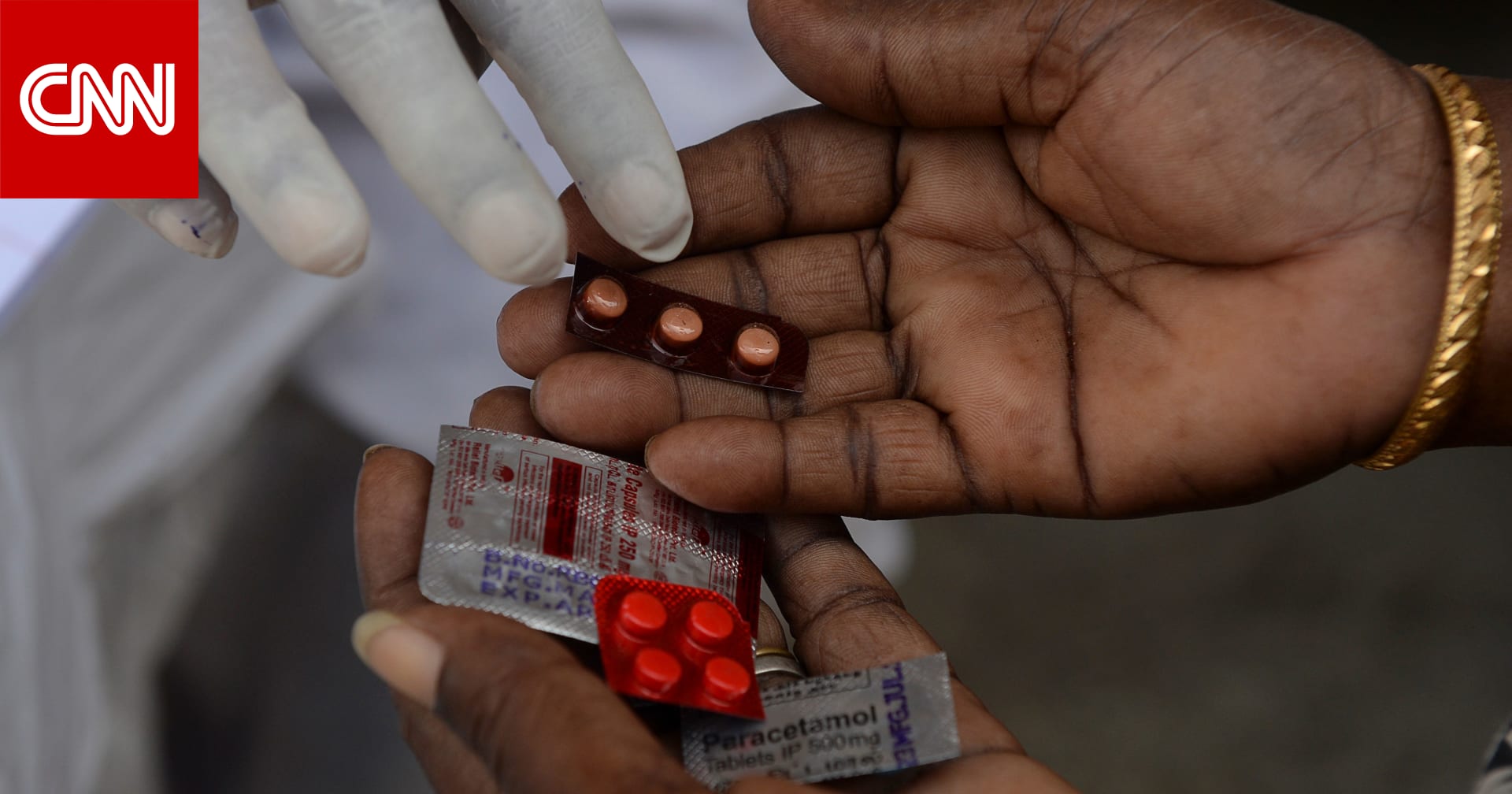
Antiviral drugs are a basic treatment for other viral infections, such as hepatitis C and HIV. One of the most important of these drugs is Tamiflu, which is widely used for its ability to shorten the duration of influenza infection and reduce the risk of hospitalization, if taken early.
Medicines developed to treat and protect the spread of viral infections in humans and animals work differently depending on the type of virus. But these drugs are designed to stimulate the immune system to fight inflammation, block receptors, prevent viruses from entering healthy cells, or reduce the level of viral activity in the body.
At a minimum, three antiviral drugs are in clinical trials. The results are expected in late fall or winter, according to Karl Diefenbach, director of the HIV division at the National Institute of Allergy and Infectious Diseases, which oversees the development of antiviral drugs.
“I think we will get answers in the next few months about the effects of these drugs,” Dieffenbach said.
The main competitor among these drugs being tested is molnopiravir from Merck & Co. and Ridgeback Biotherapeutics, which the Kelly family participated in in trials in Seattle. Two other candidates are also available, including PF-07321332 from Pfizer and AT-527 from Roche and Atea.
These drugs work to slow down the ability of the virus to replicate in human cells. In the case of “molnopiravir”, the enzyme that copies the genetic material of the virus, produces many errors that do not allow the virus to reproduce, which reduces the amount of virus in the patient, shortens the duration of the virus. inflammation and avoids the patient’s weak immune response which can expose him to serious illness or death.
So far, only the drug “remdesivir” has been approved for use as an antidote to the “Covid-19” virus. It is administered intravenously to patients requiring hospitalization and may not be widely used in the near future. Competitors, who are at an advanced stage of the events, will be available in tablet form.
And Dr Sheehan, who was involved in overseeing the stages of the clinical trial of the drug “Remdesivir”, had conducted an initial study in mice which showed that the drug “molnopiravir” could stop early disease caused by ” SARS-CoV-2 ‘virus, which causes the Corona virus. The equation was discovered at Emory University, then acquired by Ridgeback and Merck.
This was followed by clinical trials, including an early trial with 202 participants last spring, which showed the drug rapidly reduces infectious virus levels.
Merck CEO Robert Davis said this month that the company expects more comprehensive Phase 3 trial data in the coming weeks, with the option to apply for emergency use authorization from the Food and Drug Administration before the end of the year.
Pfizer also began Phase 2 and 3 trials of its product on September 1, while Atea officials await results from Phase 2 and 3 trials later this year.
Dieffenbach explained that if the results are positive and an emergency use authorization is granted to any product, distribution can begin quickly.
This means that millions of Americans will soon be able to get a daily oral medication, one tablet, for 5 to 10 days, after the initial confirmation of infection with the Corona virus.
And competition has returned to its fiercest in research and funding for oral anti-coronavirus drugs, after research efforts slowed due to the halt in the spread of the main “SARS” virus in 2003, and the Middle East respiratory syndrome virus, in 2012, according to Sheehan.
Antiviral drugs will join monoclonal antibody therapies, which are used to treat coronavirus and prevent serious illness and hospitalization. It has been possible to easily develop this type of monoclonal antibody which mimics the body’s natural response to infection, but treatment is only by intravenous injection.
Griffin stressed that antiviral pills, like monoclonal antibodies, would not replace a vaccine, but rather another tool to fight the virus. “It’s good to have another option,” he added.
The biggest barrier to the rapid development of antiviral drugs is getting enough people in the Seattle area who are not immune to participate in clinical trials within five years, Dr Days said of a test. corona positive, because this step requires hundreds of people.
Merck officials expect to produce more than 10 million treatments by the end of the year, while Atea and Pfizer have not released any information in this regard.
Source link