[ad_1]
Breaking News Emails
Receive last minute alerts and special reports. News and stories that matter, delivered in the morning on weekdays.
By Shamard Charles, M.D.
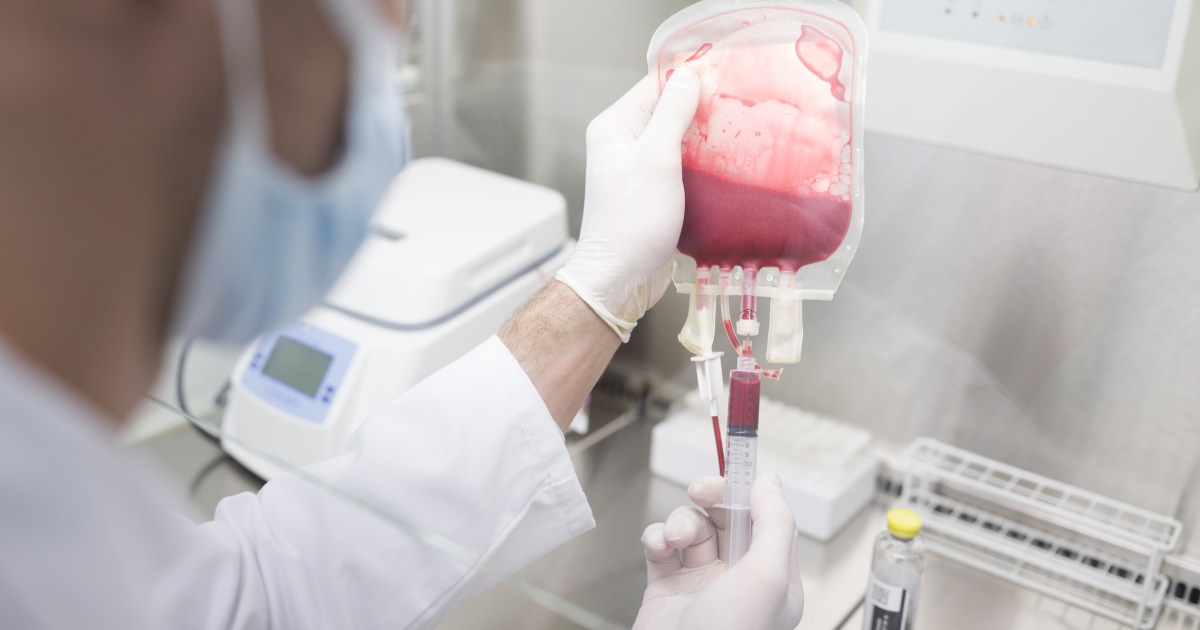
A company that billed its patients for thousands of dollars for blood plasma infusions from younger donors announced Tuesday that it had stopped treating patients after the Food and Drug Administration warned consumers against such treatments, supposed to prevent aging and memory loss.
The company, Ambrosia, said on its website that she "had stopped patient treatments". This announcement was made a few hours after the FDA issued a statement stating that there was no evidence that plasma from young donors could be used to treat dementia, Parkinson's disease, multiple sclerosis in plaques, Alzheimer's disease or post-traumatic stress disorder, as claimed by some companies.
Plasma infusions can also be dangerous, the agency added, as they are associated with infectious, allergic, respiratory and cardiovascular risks.
"We alert consumers and health care providers to the fact that plasma-based treatments from young donors have not been subjected to rigorous testing normally required by the FDA to confirm therapeutic benefit." of a product and to guarantee its safety, "said Dr. Scott Gottlieb. Peter Marks, director of the agency's Center for Evaluation and Research Biologics, said in a statement.
The federal agency noted that several companies offer plasma infusions costing thousands of dollars per infusion for various conditions. These companies can often avoid FDA drug approval processes because plasma transfusions are a well established procedure. Ambrosia, for example, was asking for $ 8,000 for a liter of blood and $ 12,000 for two for a clinical trial. The 16 to 25 year olds gave blood to consumers aged 35 and over.
Ambrosia founder Jesse Karmazin, 34, a graduate of the Stanford School of Medicine, launched the startup three years ago. In 2017, he started a clinical trial and touted impressive results, claiming that young plasma could prevent Alzheimer's disease and lower blood cholesterol levels. But he never made the results of his study public.
Ambrosia is not the only company to have marketed scientifically questionable potions to those desperate to preserve their youth.
Other medical institutions, such as the Maharaj Institute in Florida, have begun expensive clinical trials and openly discussed plans to bill patients for plasma transfusions from young donors. In fact, people planning to register said they were told they would pay $ 285,000, STAT announced in March.
"While the use of young plasma is promising and promising, rigorous trials must be conducted to ensure therapeutic benefits and safety standards for each indication of use," said Dr. Sharon Sha, neurologist. at the Stanford Health Neuroscience Center in Palo Alto. California. "I am also concerned about" paid "sites that encourage patients who are not aware of the risks and who can desperately find a cure to participate."
Plasma is the liquid part of the blood that contains clotting factors, antibodies and other important proteins. According to a small study from Stanford University, plasma infusions of young donors have slightly positive results in patients with Alzheimer's disease.
Plasma transfusions are normally administered to correct deficiencies in blood clotting in patients who bleed. Plasma is not approved by the FDA to treat other conditions, such as normal aging or memory loss.
"Plasma administration for indications other than those recognized or approved by the FDA must be performed by a qualified investigator or sponsor who has an active experimental drug application with the FDA," the FDA said.
[ad_2]
Source link
