[ad_1]
Stool transplants are experimental procedures in which doctors use the stool of a healthy donor to treat a person whose microbiome has been unbalanced.
The procedure has been very promising for the treatment of various health problems.
But now, an immunocompromised patient who has received a fecal transplant for an undisclosed reason has died as a result of the procedure and is critically ill, prompting the Food and Drug Administration to terminate several fecal transplant tests.
According to one New York Times history, both patients received fecal transplants from the same donor.
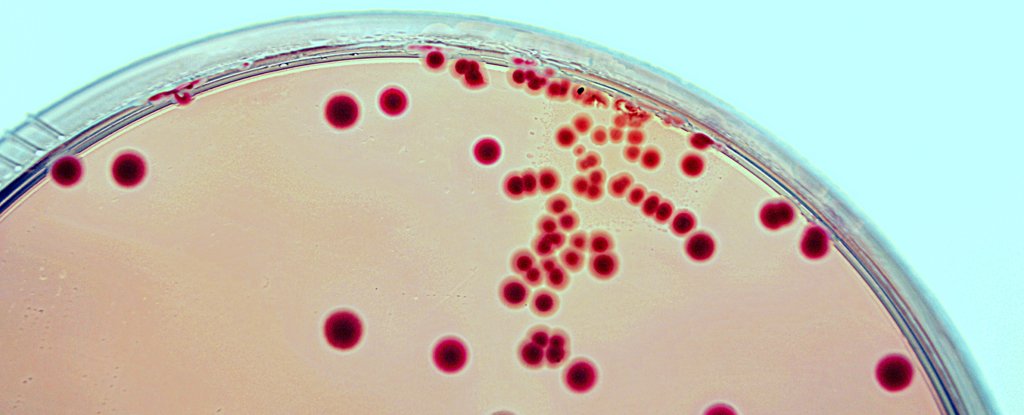
After being ill, the researchers who conducted the test analyzed the donor's samples and found that it contained E. coli bacteria that have produced an antibiotic-resistant enzyme.
Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said The temperature that the agency arrest an undisclosed number of clinical trials until the researchers who directed them prove that they have screening procedures to ensure that Stool data do not contain any dangerous organisms.
This article was originally published by Futurism. Read the original article.
[ad_2]
Source link