[ad_1]
This represents further progress in preserving a woman's fertility from the impact of cancer treatments, experts say.
"The artificial ovary will consist of a scaffold (derived from the woman's own tissue or a given tissue) associated with her own follicles," Pors writes in a email. "It's newly built, but organic."
When a woman is diagnosed with cancer, she might want to consider how best to preserve her fertility, since radiotherapy and chemotherapy, which are commonly used as cancer treatments, often destroy the function of her ovaries. [19659005] Two methods of preserving fertility are available. One option is to remove and freeze some of her eggs so that after her cancer treatment and that she is ready for a child, she can attempt in vitro fertilization. The second technique, commonly referred to as ovarian tissue transfer, is to remove ovarian tissue prior to treatment, freeze and re-implant after treatment.
This second option is used less often than the first because the ovarian tissue is removed before the treatment contains malignant cells and, once implanted, the cancer could be reintroduced into a woman's body.
eliminate the possibility of reintroducing cancer into the tissue of origin.
Pors and his co-authors a scaffold of ovarian tissue that is free of cancer, they could seed it with early stage follicles frozen, which could then develop and ripen naturally. In the end, this could restore a woman's fertility.
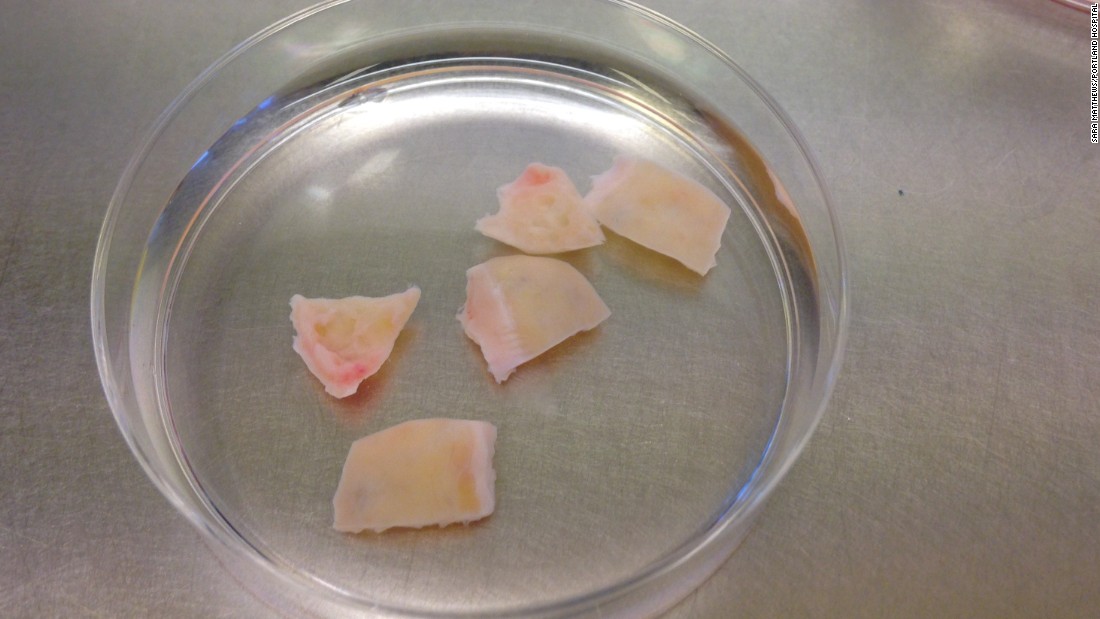
First, they experimented on human tissue donated by patients trying to preserve their fertility. Using a three-day chemical process, the researchers eliminated all the cells that could contain cancer tissue.
Follicles, unlike ovarian tissue cells, do not contain cancer, Pors said. "The follicle is formed during fetal life [when no cancer is present] and is surrounded by a basement membrane that does not allow cancer cells to enter," she said.
The chemical process has left a "decellularized scaffold" consisting of proteins and collagens, the normal material that connects the cells. The researchers seeded this structural matrix with early-stage follicles. As expected, the equipment allowed communication between the cells and allowed them to grow.
This development occurred in vitro, or outside the body.
Then, the researchers experimented with mice and transplanted a decellularized and seeded matrix in animals. This supported the survival and growth of the early-stage follicles.
"It is the first time that isolated human follicles have survived in a decellularized human scaffold," concluded Pors and his co-authors
. -Accept, Pors said that he could offer a new strategy in the preservation of fertility without the risk of cancer recurrence.
More Research Necessary
He added that the new technique only transplanted eggs and follicle cells (seeded into a matrix) into the uterus.
"This could give rise to a problem in itself, however, because the surrounding ovarian cells left behind might be needed for the ovary to function fully," noted Brison.
While this approach may work, he concluded that "it is not possible to say whether the data from this research group have been peer-reviewed by the scientific community and published in a scientific journal. "
"At this point, many questions remain," said Anderson, who was not involved in the research. "But it's certainly a promising approach."
About 2% of women of childbearing age who have cancer and treatment may lose their ovarian function – and therefore their fertility. They are at risk of premature menopause, and although the use of hormone replacement therapy and their own cryopreserved eggs allow some of these women to become pregnant, their natural hormones and natural fertility will not be renewed.
For young cancer patients desirous of preserving their fertility, the transfer of ovarian tissue can restore menstrual cycles and allow the woman to get pregnant "in the old" – since hundreds of years ago. Eggs remain intact within the follicles – Have a huge advantage over freezing some eggs.
Source link


