[ad_1]
On October 5th, the US Food and Drug Administration (FDA) authorized the marketing of a new hearing aid capable of amplifying sounds for people 18 years of age or older with mild to moderate perceived hearing loss. .
It is the first hearing aid approved by the agency that allows users to adapt, program and control the hearing aid on their own without the assistance of A health care provider. Xinhua news agency reported.

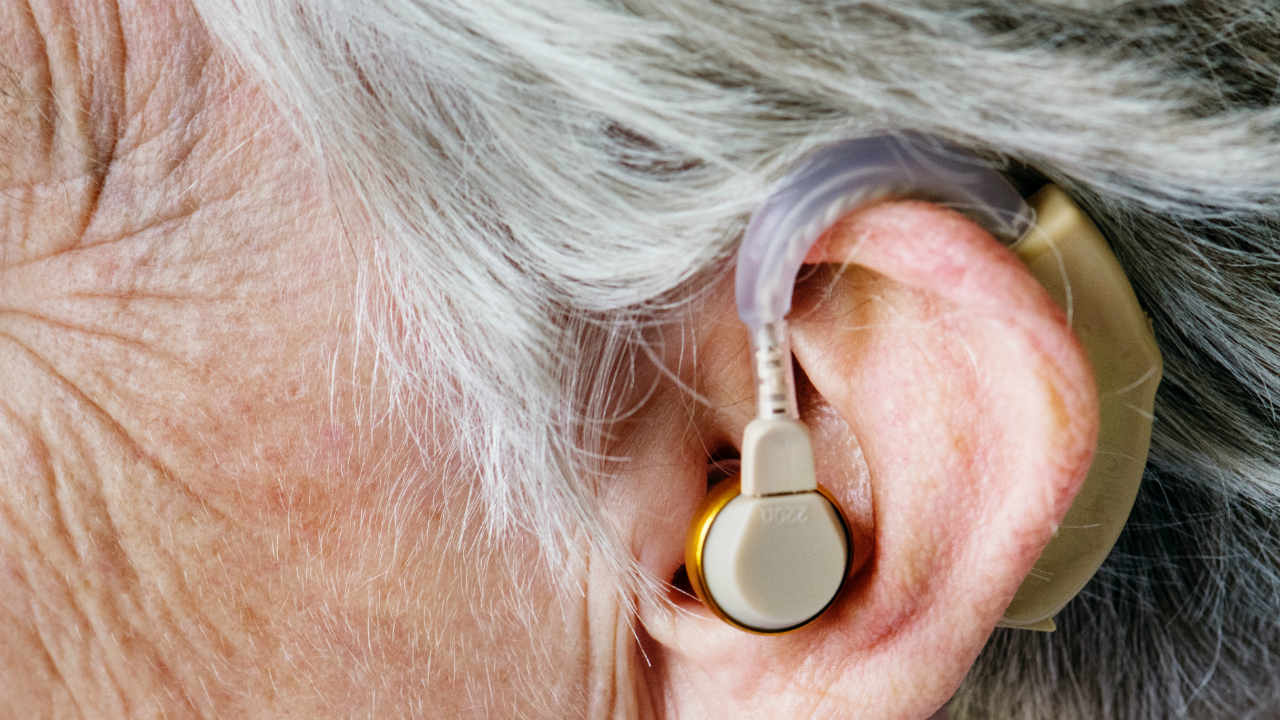
Image of representation.
The loss of hearing, permanent or temporary, can be caused by aging, exposure to loud noises, certain medical conditions or other factors. People with permanent hearing loss can use hearing aids to help them hear speech and sounds.
According to the FDA, the Bose hearing aid is a wireless hearing aid suitable for air conduction, adapted to the user.
Air conduction hearing aids can pick up sound vibrations via one or more microphones and the signal is processed, amplified, and delivered via an earpiece placed in the ear canal.
Patients can adjust the hearing aid via a mobile application on their phone. This technology allows users to customize their hearing instrument settings in real time and in real-world environments without the assistance of a health professional.
The FDA has reviewed data from clinical studies of 125 patients, showing that the results with self-adaptation of the device are comparable on average to those obtained with a professional adaptation of the same device with regard to the level of amplification selected, the test of speech in noise and advantage.
[ad_2]
Source link