[ad_1]
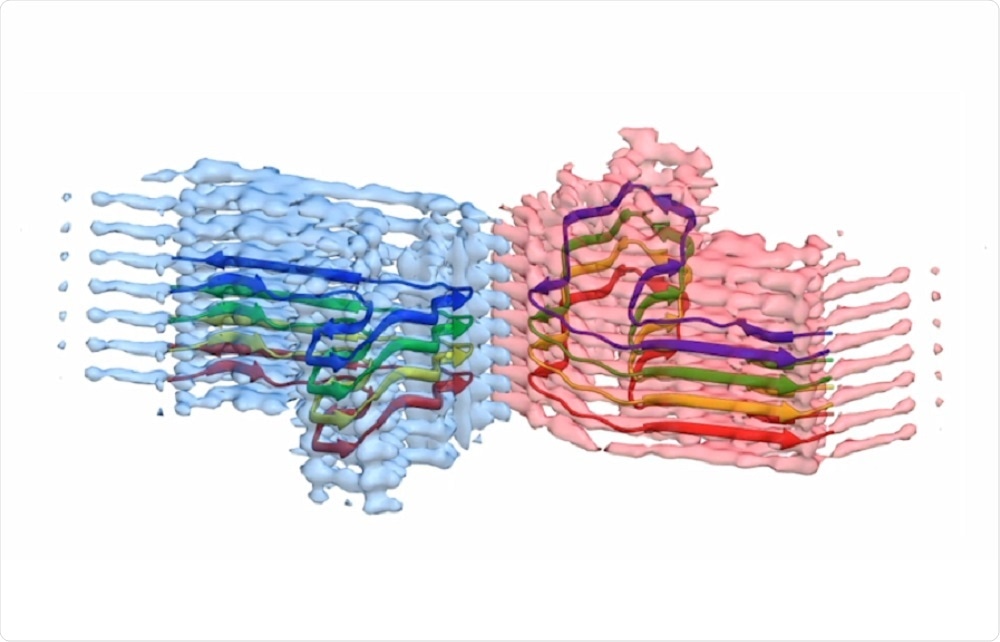
Scientists at the University of Leeds have used cryo-electron microscopes to reveal the structure of amyloid – the abnormal protein that accumulates in the body and causes diseases such as Alzheimer's disease, Parkinson's and Huntington's disease.
 Credit: University of Leeds
Credit: University of Leeds
"Revolutionary" high power microscopes use electrons rather than light to visualize the shape of the samples at a resolution close to the atom.
It is only recently that British scientists have had access to these scientists, although fewer than 25 of them are in universities and research institutes in the country.
The University of Leeds houses two microscopes, which are the only two of its kind found in northern England.
They have already proved invaluable in a number of projects, but the current achievement is the most successful ever.
As less than ten high-quality images showing the structure of amyloid are available to study worldwide, the contribution of prospects therefore represents a major step in understanding the formation and disease of protein aggregates.
The five-year project was led by structural molecular biology experts Sheena Radford and Neil Ranson, who also collaborated with Professor Bob Griffin of the Massachusetts Institute for Technology.
As reported in the newspaper Nature Communicationsthe images and 3D structures generated by the microscopes have shown that the aggregates form long twisted fibers.
The protein they've studied – β2-microglobulin – generally plays a role in the immune system, but can aggregate in amyloid fibers causing pain in long-term dialysis.
Fibers can also cause osteoarthritis when they lodge in a person's joints.
Over the past six decades, since the creation of the first electron microscopic images of amyloid, scientists have gone from processing low-resolution fuzzy images to our ultra-sharp 3D images and structures, thanks to modern advances in cryo-electronic microscopy. "
Professor Sheena Radford, Principal Investigator
She hopes that now the team can visualize the exact location of each node and point to the protein, it will be possible to develop compounds that will bind or disrupt it, so as to be able to explore the role of the protein in the disease. .
We have used electron cryoscopic microscopy not only to discover the shape and structure of amyloid proteins, but also to determine how they develop and mingle with each other, such as the supports of a rope, to form larger assemblies. This knowledge will be crucial to knowing how to manage them. "
Professor Sheena Radford, Principal Investigator
Then Radford intends to identify and develop inhibitors controlling assembly of amyloid fiber proteins and the Wellcome Trust has agreed to provide nearly £ 2 million to support this next step.
sources:
The 10-foot high microscopes help fight the worst diseases in the world.
The structure of a β2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism
[ad_2]
Source link