
[ad_1]
Scientists have used the CRISPR-Cas9 gene edition to alleviate some autism symptoms in mice with some form of fragile X syndrome, the most common cause of autism spectrum disorder.
Mice with Fragile X syndrome were treated with CRISPR-Cas9 to eliminate a gene involved in obsessive behavior and exhibited much less repetitive excavation behavior. A similar approach using the CRISPR-Gold technique could be used to treat the exaggerated repetitive behavior characteristic of other autism spectrum disorders. Video by Roxanne Makasdjian and Stephen McNally, with video from UT Health Science San Antonio.
Using gold nanoparticles to administer the Cas9 enzyme in the brain – a technique developed at the University of California at Berkeley, and called CRISPR-Gold – researchers were able to edit the gene for 39, a neurotransmitter receptor and reduce repetitive behavior. Fragile X syndrome (FXS).
Because exaggerated repetitive behaviors are common in autism spectrum disorders, the effective reduction of these behaviors in FXS mouse models demonstrates the potential application of this technique to other types of autism for which genetic cause is known, say the researchers.
"There are still no treatments or cures for autism, and many clinical trials of small molecule-based therapies targeting the autism-causing proteins have failed," he said. Hye Young Lee, Adjunct Professor of Cellular and Integrative Physiology at the University of Texas Health Science Center in San Antonio. "It is the first case where we have been able to edit a causative gene for autism in the brain and show the rescue of behavioral symptoms."
According to the researchers, CRISPR-Gold has many advantages over other ways to put Cas9 in the body, such as using viruses.
"The really compelling thing about this article is that Hye Young was able to show that if you injected CRISPR-Gold into the brain, you could eliminate pathogenic genes and see quite significant behavioral changes," said Niren, inventor of CRISPR-Gold. Murthy, a UC Berkeley professor of bioengineering. "It's the first time anyone has shown it with a non-viral delivery."
Those with autism spectrum disorders have problems interacting with other people as well as exaggerated repetitive behaviors, such as tipping and flapping. Although ASD appears to have various causes, including multiple genetic mutations, monogenic disorders such as FXS are a simpler way to explore the causes and potential treatments. While autism spectrum disorders affect more than one percent of all children, FXS is rare, occurring in one in every 4,000 and 6,000 girls.
The results will be published online on June 25 in the monthly journal Biomedical engineering of nature.
Researchers have injected gold nanoparticles (upper right) into the brains of fragile brittle X-ray mice to alter DNA (scissors) and eliminate a neurotransmitter receptor that reduces the exaggerated repetitive behavior characteristic of autism spectrum disorder. Health Sciences Center of the University of Texas at San Antonio.
The new study is the first demonstration that the Cas9 protein can be transported into the brain to knock out a gene and have therapeutic effects. While other researchers have inserted genes for Cas9 into neurons via viruses such as the adeno-associated virus, problems arise because the gene continues to express the Cas9 enzyme, leading to random cutting. Other genes. CRISPR-Gold carries the Cas9 complex itself – the purified Cas9 protein and the RNA guide – directly into the cells, where it cuts a few times and then disappears.
"If you inject CRISPR DNA using a virus, you can not control the amount of Cas9 protein and the guide RNA are expressed, so the injection via a virus has a potential problem" Lee said. "I think the CRISPR-Gold method is very cool because we can control the amount we want to inject and that probably minimizes the side effects of using CRISPR, for example out-of-target effects."
The technique opens the door to treating conditions ranging from opioid addiction and neuropathic pain to schizophrenia and epileptic seizures, said Murthy.
The amortization of an over-excited brain
In the experiment on mice with FXS, the researchers injected CRISPR-Gold carrying the Cas9 complex into the brain striatum, a region known for mediating habit formation, including that related to repetitive behaviors common to ASD. Cas9 targeted an excitatory receptor, the metabotropic glutamate receptor 5 (mGluR5), which is involved in communication between neurons and is known to be dysregulated in FXS. By disabling the mGluR5 gene, researchers were able to mitigate the exaggerated signaling between cells and reduce repetitive behavior.
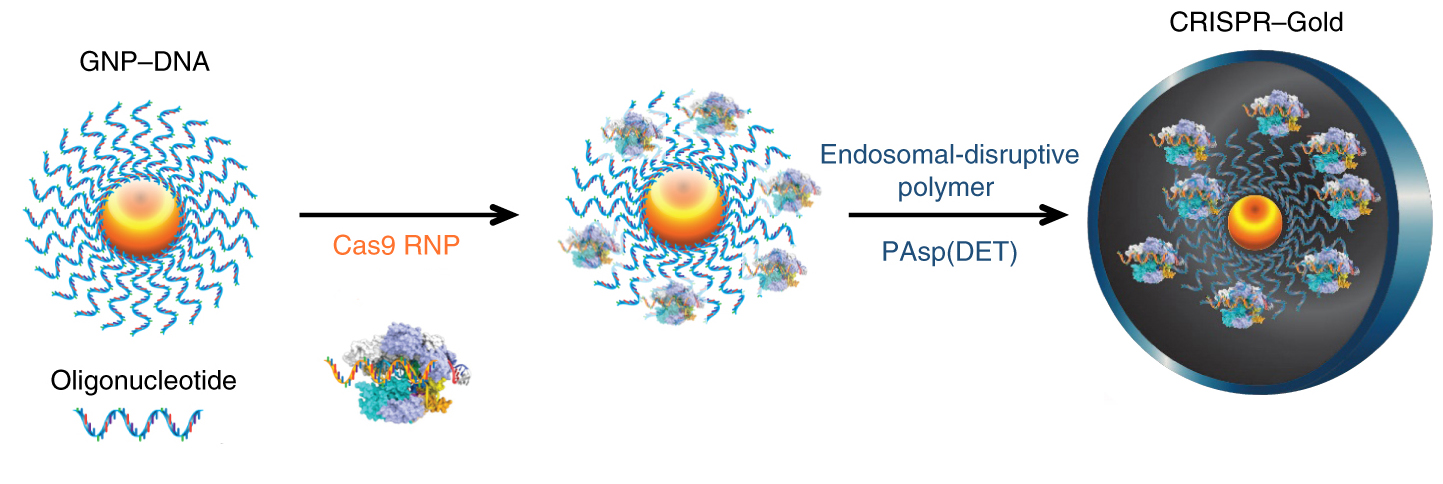
To make CRISPR-Gold, a CRISPR distribution system, a gold nanoparticle is coated with a forest of DNA oligonucleotides and decorated with Cas9 ribonucleoproteins, protein complexes, and proteins. RNA guide. It is then coated with a polymer that helps the particle to be absorbed by the cells, in this case the neurons, astrocytes and microglia in the brain.
"Prior to this experiment, we did not know if the mGluR5 receptor in the striatum was specifically involved in exaggerated repetitive behavior, it is an important biological finding of our study," Lee said.
In mice with FXS, repetitive behavior included obsessive digging and periodic air jumps. Digging was reduced by about 30 percent, while the jump dropped by 70 percent. About 50% of the mGluR5 genes in the striatum were edited, which reduced the number of receptor proteins by almost half.
Pharmaceutical companies have tried to inject small molecule drugs into the bloodstream to block the same receptor, but if some reduction in repetitive behavior was noted, the mice did not respond to subsequent treatments, becoming apparently tolerant.
The promising CRISPR-Gold system was developed by Murthy, which focuses on the administration of drugs and the development of new antibiotics. The technique uses gold nanoparticles covered by a forest of DNA chains that contain Cas9 molecules, which are a combination of a gene-cutting enzyme and a guide RNA that houses the mGluR5 gene. The package is encapsulated in a polymer that helps in getting into the proper cells.
Last year, Murthy and his colleagues demonstrated that CRISPR-Gold could carry Cas9 into muscle cells and replace a mutated gene with a normal gene to enhance strength in Duchenne muscular dystrophy mice.
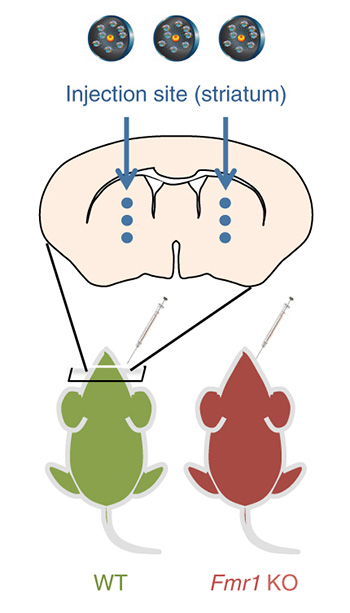
CRISPR-Gold bearing Cas9 complex was injected into the striatum of the mouse brain (red) that had been created with some form of fragile X syndrome. As a control, normal mice were also injected with CRISPR-Gold.
The new paper proves that CRISPR-Gold can successfully get Cas9 into a variety of cells in the brain.
"We showed in this article that we were also able to edit non-neurons: microglia, which are part of the brain's immune system, and astrocytes, which support neurons," Murthy said. "We have edited them more efficiently than neurons, and these can play a very important role in many diseases."
In 2016, Murthy's lab created a start-up, GenEdit, to produce and test CRISPR-Gold therapies for a variety of genetic diseases. According to GenEdit CEO and UC Berkeley Ph.D. Kunwoo Lee, who is currently working out of the QB3 garage on the UC Berkeley campus, the successful assembly of brain cells opens the door to the treatment of many disorders.
"CRISPR-Gold can be used to treat a variety of genetic diseases, such as Huntington's disease," Lee said. "But this is not limited to monogenic diseases, it can also be used against polygenic diseases, once we have understood the entire network of genes involved."
GenEdit explained how to ship CRISPR-Gold particles long distances – from Berkeley to San Antonio – and make them reproducible, eliminating a bottleneck that has limited the translation of many other nanotechnologies.
Hye Lee Young, Kunwoo Lee and Murthy are now working to develop CRISPR-Gold particles that can be injected directly into the central nervous system through the spinal cord, thus avoiding opening the skull and injecting directly into the brain. Hye Young Lee is optimistic that it could be as effective as intracranial injection into the striatum to reduce repetitive behavior, and perhaps even solve some of the social interaction problems of ASDs.
UT Health San Antonio's Bumwhee Lee Postdoctoral Fellow and GenEdit's Kunwoo Lee are the co-first authors of the article. Other writers are technician Shree Panda, postdoc Vladislav Bugay and associate professor Robert Brenner of UT Health San Antonio, high school student Rodrigo Gonzales-Rojas, and Hyo Min Park and Anthony Chong of GenEdit.
The work was supported by the California Institute for Quantitative Biosciences (QB3) at UC Berkeley, the National Institutes of Health (R01EB023776) and an award from the University of Texas Rising STARs at Hye Young Lee . Kunwoo Lee, Park and Murthy are co-founders of GenEdit Inc.
ASSOCIATED INFORMATION
Source link