[ad_1]

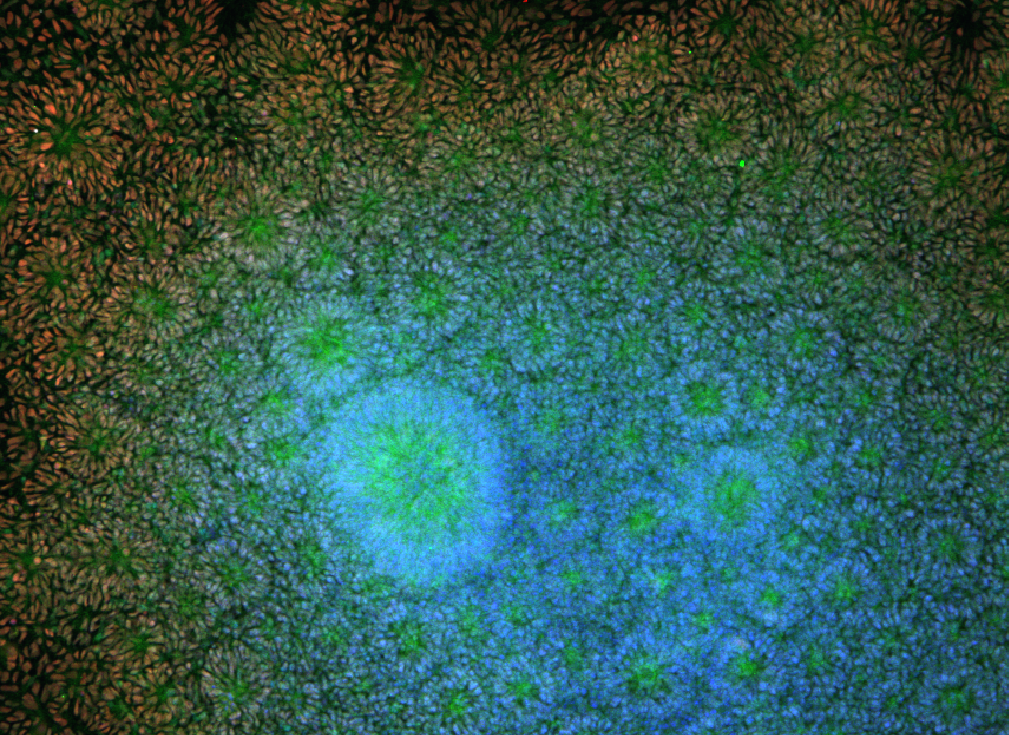
William Murphy can be used in "tissue-on-a-chip" systems to screen new drugs for toxicity and effectiveness. Image: Angie Xie, Mike Schwartz, William Murphy
There was a time when Eric Shusta was more connected to the local butcher shop than you might expect.
To learn more about the blood-brain barrier – the cellular wall between the bloodstream and the protected confines of the brain – and how to trick the body into the foreign molecules (like drugs for neurological disease) through the barrier's gates, Shusta needed brains.
"We were trying to model that layer of cells in the brain in a petri dish, and we were using primary brains," says Shusta, a University of Wisconsin-Madison. professor of chemical and biological engineering. "The issue is, they're not human brains."

Eric Shusta
But engineers are beginning to make use of a new tool, which is one of the following:
One of those researchers, Sean Palecek, chemical and biological engineering professor, was right down the hall from Shusta. Palecek was exploring pluripotent stem cells developed by UW-Madison researcher James Thomson as a way to study heart muscle and other tissue. Shusta and Palecek each of these end-up cells in the endothelial cells, the type that makes up much of the blood-brain barrier.
"The graduate students figured out how to differentiate these pluripotent stem cells into endothelial cells that had many brain-like characteristics – the transport systems that move things through the cells, the tight way they're packed together in the vasculature brain – to really recapitulate "Shusta says." The important aspects of a human brain, "Shusta says.

Sean Palecek
"We told them it would not work, but they figured it out," he adds. "That's why you always listen to the graduate students."
William Murphy, Professor of Biomedical Engineering and Orthopedics and Rehabilitation.
Since Murphy joined the UW-Madison faculty in 2004, his lab has worked to (among other things) spur the regeneration of tissue inside and outside of the body. Before stem cells, he would typically start with adult cells from humans. But some human tissue is very hard to get. Living brain and liver donors are understandably scarce. And lots of fully developed cell types do not like to divide and multiply for researchers.
"So, even if you can find tissue, it's not like you can grow in large quantities from an adult cell," Murphy says. "That's one of the primary benefits of stem cell discovery. Now you have this unlimited feedstock to generate human cells for tissues. "
That offers researchers an opportunity to work at a scale that many alternative sources of human or animal tissue could not.

"From one mouse brain I could get six tiny experiments. I could look at two variables in triplicate, "Shusta says. "From stem cells, it's scalable. We can do hundreds of years of growing and growing plants. "
Much of the promise of the stem cells used in engineering labs comes from their pluripotency, the ability to turn into other types of cells. Since Thomson published his embryonic stem cell stem cell research in 1998 (and since he and others figured out about 10 years later how to coax certain types of human cells back into a pluripotent stem cell state), scientists have worked out how to spur stem cells into much of what labs need to try.

William Murphy
"For most of the cell types that would be protocols – cardiac muscle, neurons, microglia, blood vessels, retinal cells," Murphy says. "A significant number were discovered here at UW-Madison."
Murphy also studies the environment around stem cells, and the way it affects how many cells differentiate into new cells.
"They have this remarkable ability to self-organize into human tissue structures, and even human-type organ structures," Murphy says. "But they do not self-organize the right way under all conditions."
The factors that guide that organization are chemical and physical. Getting the right cell receptors and proteins can be as important as figuring out how soft or hard or remodel-able. Find the right combination of factors, the right microenvironment, and it's possible to nudge stem cells in the direction of building bone and tendon and blood vessels, and more.
"It's a very rare environment in which people will self-organize," Murphy says. "But they do not, the advantage is that it's simple. You got the cells in that environment, and when you come back they've done it. "
Those kinds of environments are even rarer in the lab. But a good place to take a look at how to create a home environment.
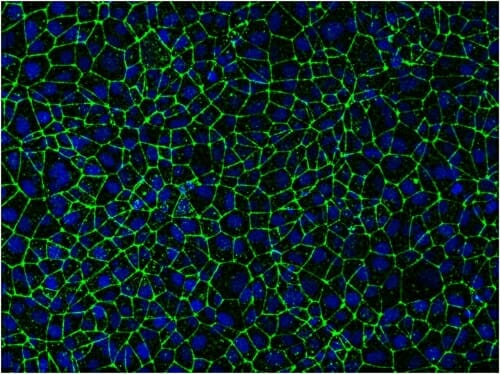
Stem-cell-derived endothelial cells packed tightly together give researchers a way to study the blood-brain barrier, including opportunities to try out ways to deliver drugs to the brain through the barrier's own cellular gates. Image: Eric Shusta and Tongcheng Qian
"A lot of what we do is motivated by what we know about the way cells develop in the body, and how we can recapulate that process in a dish," says Palecek.
Palecek's lab is working with the UW-Madison cardiologist on the development of heart cells called cardiomyocytes. That success came in 2012, and the effort made to refining production methods.
Palecek says, "Now we're working on how to produce them on a clinical scale. "We can make a million cardiomyocyte cells. A person who has a heart attack typically loses a billion cells. So, if Tim Kamp wants to start a clinical trial, he is going to need a lot of cells. "
The stem cell field is building infrastructure to make it even easier to set up a new cell experiments.
Like many stem cell researchers, Shusta is excited by the opportunity to study the development of genetic diseases. Pluripotent stem cells, which can be done by taking cells from a person with the disease. study the disease.
"We very quickly became the top institution in the stem cell engineering area. That was a field that did not exist, and we built it. "
William Murphy
Shusta and Clive Svendsen, a UW-Madison neurologist trained, had this rare condition with a rare neurological condition called Allan-Herndon-Dudley Syndrome, the first model of the disease-in-blood-brain-barrier cells in which its progression and potential treatments could be Studied.
But it takes a lot of time and resources to work through that process for each disease or disorder.
"In many cases, you do not need to do that work anymore, because they are efforts funded by the federal agencies where they are banking these materials – stem cell lines from a whole host of control and disease subjects," Shusta says.
"If I want to work on Alzheimer's disease, I'm able to find that I have a genetically modified cell line with a high risk allele of Alzheimer's disease, with allele with risk, and with allele with risk diminished, "Shusta says. "That's an important head start."

Randolph Ashton (right)
Biomedical engineering faculty at UW-Madison is at work on new platforms to study disease. Randolph Ashton's lab is using stem cells to grow neural tubes, a precursor of the spinal cord. David Beebe is trying to model a form of vasculitis, which causes chronic inflammation of blood vessels. Krishanu Saha has used the gene editing tool CRISPR to correct disease-causing mutations in stem cells derived from patients. Gene editing could be used to add genetic disorders to stem cells, broadening their utility to a platform for the progression of disease.
Another more of stem cells' ability to repeat in a dish of the world
"Every major pharmaceutical company in the world has a stem cell program," Murphy says. "They use stem-cell-based assays for the discovery of new drug candidates and sometimes for testing a drug on a human cell population."
In many cases, these tests are supplemented by testing in animals, although stem-cell-based processes typically have a look at the toxicity or efficacy of a drug on one type of cell or tissue at a time.
Murphy's lab is working to make this tissue-on-a-chip testing approach to cancer treatment, starting with a type of skin cancer, metastatic melanoma, which is likely to spread to places like the brain and liver .

Krishanu Saha (right)
"We can take metastatic melanoma from you, add it to your tissue and your brain, and try to understand how your metastatic melanoma will behave," he says. "Is your cancer particularly likely to attach to the liver? If it is, which drug candidates might you be interested in this particular melanoma? "
This personalized version of medicine can be used as a single cell or tissue type. It's much harder to recreate the complexity of the body and systems that work together in animals.
"If you want to test the effect of a chemical drug or toxin on the brain, then you really need to have a chemical-related compound that might be processed in the liver and affect the brain," Murphy says. "So, a model needs to have a brain and a brain connected by some kind of blood supply. And then if you're going to do that, you might also need to have an immune system present, because the immune system might have an important effect on the process. "
Engineering ways to become more mainstream, but the stem cell has had a long way since 1998.
Palecek says, "Where we've been able to go in 20 years is big, and every year there is more opportunities," Palecek says. "We're still at the exponential growth phase."
UW-Madison, according to Murphy, who was director of the university's Stem Cell and Regenerative Medicine Center until earlier this year.
"We very quickly became the top institution in the stem cell engineering area. That was a field that did not exist, and we built it, "he says. "Now it's a very popular nationally, and we're a leading hub for it."
Source link
Share via Facebook
Share via Twitter
Share via Linked In
Share via Email