[ad_1]
Several months after the US Food and Drug Administration approved for the first time the use of a marijuana drug, the drug became available in the United States on Thursday.
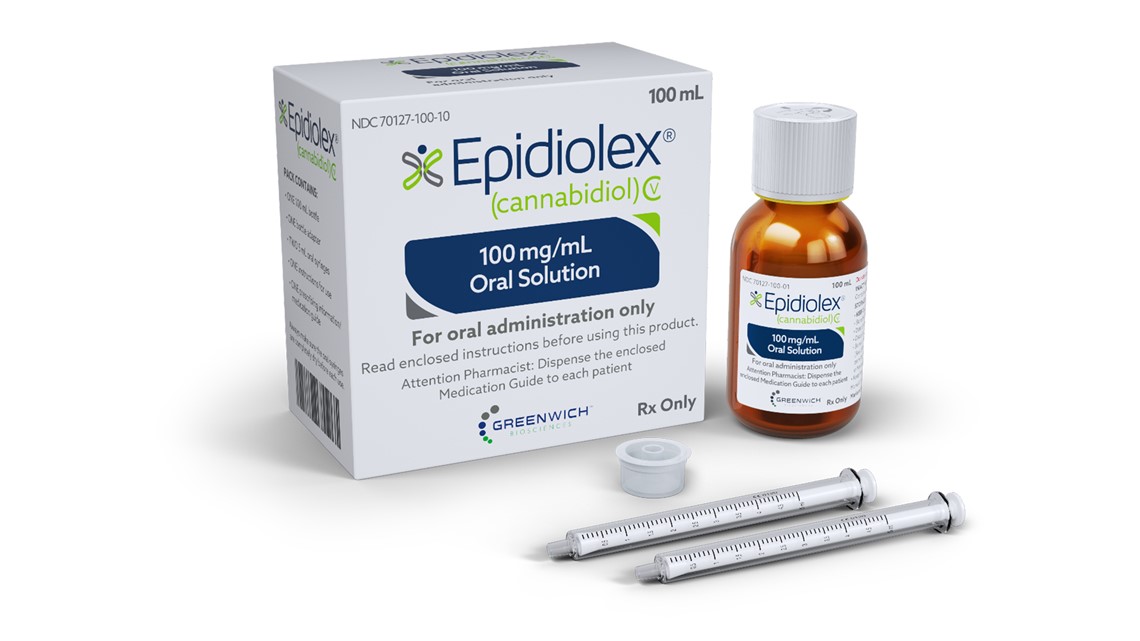
Epidiolex will be used legally to treat two types of serious and rare epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, with the compound cannabidiol, also known as CBD, present in hemp and marijuana. Epidiolex is the first approved treatment for Dravet syndrome, according to the FDA.
"We are excited to announce that Epidiolex is now available on doctor's prescription as a new treatment option for LGS patients and Dravet syndrome, two of the most common forms of epilepsy." difficult to deal with, "said Justin Gover, Executive Director. Officer of GW Pharmaceuticals, the company that developed the drug, in a statement.
Although CBD is extracted from sativa marijuana plants, it does not produce the effect usually associated with marijuana because it contains no psychoactive ingredient, THC. Instead, CBD is often used in the form of oil as a means of relieving anxiety without a high effect.
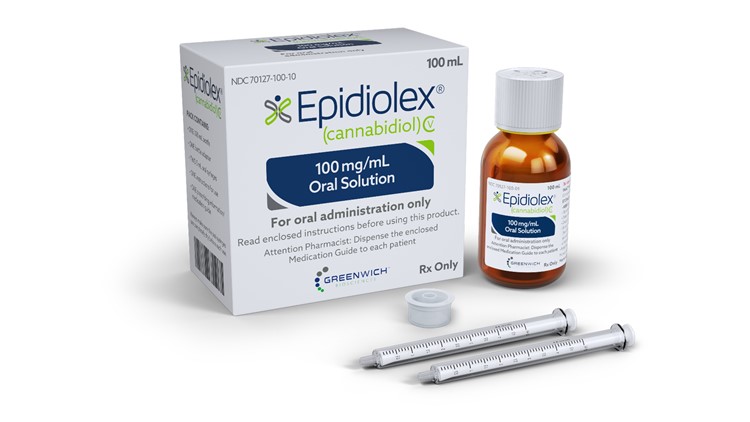
Epidiolex is the first FDA-approved marijuana-based epilepsy drug.
GW Pharmaceuticals
"This approval is a reminder that the implementation of valid development programs that correctly evaluate the active ingredients contained in marijuana can lead to important medical therapies," FDA Commissioner Scott Gottlieb said in a statement. approval of the drug in June.
Epidiolex has been effective in reducing seizure frequency in patients with both syndromes in clinical trials. Since Lennox-Gastaut syndrome and the Dravet synonym both appear in infancy, Epidiolex is legal in treatment for patients aged two years and older.
Both syndromes are characterized by severe and uncontrollable convulsions, resulting in a form of learning disorder, such as a language and motor impairment.
For families with loved ones suffering from these crises, the approval of Epidiolex offers "an indispensable hope," said Christina SanInocencio, executive director of the LGS Foundation, in a statement. But even with CBD oil, most people with SLS "will continue to have epileptic seizures for life," she added.
According to the FDA, some of the side effects reported in clinical trials include drowsiness, sedation, lethargy, elevated liver enzyme levels, decreased appetite, diarrhea, rash, weakness, insomnia, poor sleep quality and infections.
The CBD is currently considered an Schedule I drug because of its relationship with marijuana. Trials have been conducted to examine the potential for CBD abuse.
The CBD industry is worth $ 200 million, according to the CBD summit. The use of CBD in the treatment of other diseases and disorders, such as posttraumatic stress disorder, is being tested.
Contributor: TEGNA Staff
© 2018 USATODAY.COM
[ad_2]
Source link