[ad_1]
Last spring, a few weeks before Aimovig, the new migraine drug, was commercialized in the market, phone calls and emails began to accumulate. Patients from across the country have contacted the UCSF Headache Treatment Center, eager to receive the medication as soon as it would be available.
"We have a lot of very good treatment opportunities coming up today," said Dr. Nina Riggins, a neurologist at the UCSF Headache Center. "Patients ask us all the time to give our opinion. But we are having problems all the time. "
In the United States, about 1 in 7 people suffer from migraines, according to government statistics. And up to 3 million people have chronic migraines, which means that they have 15 days or more of migraine per month.
For those who suffer from the worst disease, the disease can be disabling. This can cause not only intense pain, but also intense nausea, fatigue, sensitivity to light and sound and some kind of brain fog. For some chronic migraine patients, the symptoms prevent them from working and have a significant impact on their ability to perform even simple tasks, such as doing grocery shopping or visiting friends.
A therapy that could move their pain from 8 – on a scale of 1 to 10 – to 4 – would change their lives.
"An 8 may be that I can not stop throwing up. It's like I'm a raw nerve and any sensation, any noise, any smell would be hard, "said Rebecca Brook, 47." I could function like most people at 4 years old. If I had a 4, it's not a problem. "
For years, his UCSF doctors had announced the arrival of new drugs. By the time Aimovig was approved, "I felt desperate to get it," Brook said. "It was like, let me start my life."
His insurer refused to pay the drug, administered by monthly injection at a cost of about $ 575 per dose. However, drug developers, pharmaceutical companies Amgen and Novartis, predicted that some insurance companies would not pay for the drug initially and would set up a program to offer patients this drug at low cost or no cost for a year – and that's how Brook finally got the drug. She has been there for three months now.
Brook still suffers from migraines – a few rare ones, in fact – but she sees signs of improvement. In October, she did not need to take the medication she needed to treat particularly severe headaches. The pain was so insignificant that she dared leave her home in Berkeley to do some shopping in San Francisco.
"I was exhausted when I got home, but I did not have a headache," she said. "And I was like, oh my God, that did not happen for decades."

In November, things were even better: she spent eight days without needing her medicine for headaches. But she still hesitates to give credit to the new drug, even if the results look promising.
Brook has been suffering from migraines since she was a teenager, and she has never found a treatment that will allow her to reduce her pain steadily or that has worked for more than a few months. She knows that she could feel a placebo effect now. Or that the drug is helping right now, but eventually will fail.
But almost as stressful: and if the drug is effective? What will happen if his insurer never covers it and can not afford it once the drug company stops paying?
"All the other treatments supposed to help migraine sufferers, I failed," said Brook. "And then I had this experience, where for two days something was going on. And I thought, "Oh my God, are they going to take my Aimovig?"
Insurance providers have announced that they are reviewing Aimovig and other new medicines and devices recently released on the market. They note that although the treatments have been approved by the FDA, this does not necessarily mean that they are necessarily useful – or profitable – for most migraine sufferers.
Drug treatments all cost about the same price and act by interfering with the action of a protein called CGRP associated with migraines. Aimovig was the first to get approval in May. Two other drugs, Emgality and Ajovy, were approved in September.
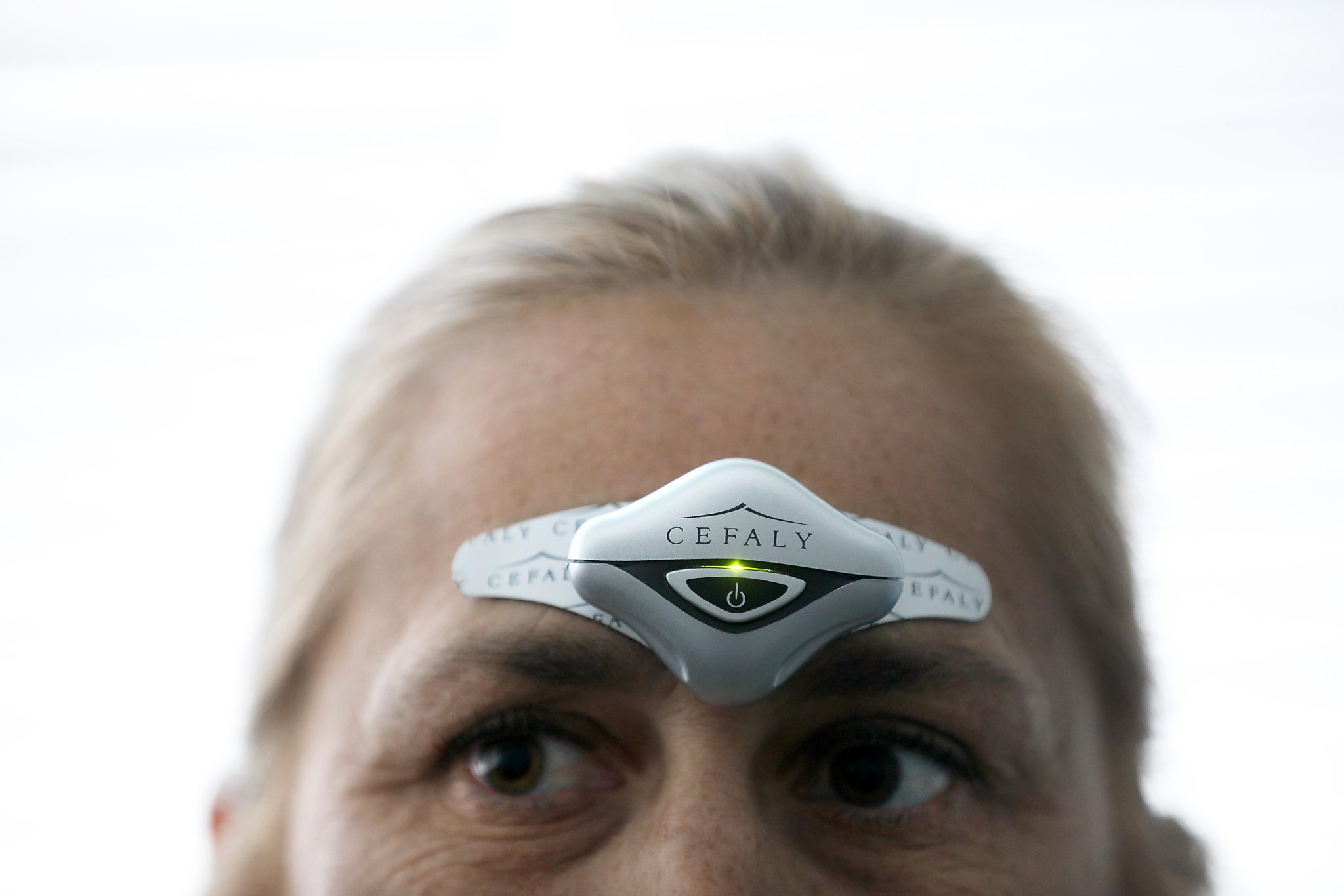
New medical devices approved in recent years have also increased treatment options for people with severe chronic migraines – but they are also rarely covered by insurance. Devices are wearable instruments that patients place on their foreheads or the back of their heads to stimulate nerves known to be affected by migraines.
The new drugs are all designed to prevent migraines and not to treat them once they have started. The devices are intended for prevention and treatment – some for one or the other and some for both.
For decades, patients have used a mix of medications that are not specifically designed to treat migraines, but that can help some people, including antidepressants and anti-epileptic drugs, or drugs to treat high blood pressure. Botox, a neurotoxin used to reduce wrinkles, is a particularly popular preventive treatment for people with chronic migraines.
These drugs only work for some patients, and even those for whom they are effective, they tend to lose power over time. In addition, they can have side effects that make them unusable for some patients.
According to clinical trials, new anti-CGRP drugs seem to have fewer side effects so far. And doctors and patients hope they will be more effective because they have been specifically designed to act on a known migraine trigger.
"These older drugs, I do not want to dismiss them. We always use them. I'm grateful to have them, "said Riggins. "But we can offer several new things these days, which is extremely exciting. In particular, drugs (anti-CGRP) really change the game. "
New therapies do not cure and will not work for all patients. In clinical trials, up to 20% of patients, known as "super responders", were able to prevent almost all migraines during Aimovig treatment. But less than 50% have seen their number of migraine days halved, a goal generally applied to migraine medications. And some patients had no effect of the drug.
Similar results have been reported for other new drugs.
Dr. Robert Cowan, director of the Stanford Headache Program, said his office has also received requests for payment for new drugs from patients across the country. But he is not as optimistic as some of his professional colleagues about their effectiveness.
He is not convinced that the drugs will work equally well for his more serious patients than for those participating in clinical trials, who tended to be a generally healthy group, apart from frequent headaches. He is also concerned that the drugs may have undesirable effects that have not been detected in several thousand patients tested so far.
"I'm kind of a loyal opposition at this stage," said Cowan. "I just think there is a lot of hype around these drugs. Maybe they are awesome. I hope they are awesome. But I do not have enough information yet. "
Cessa Marshall, 51, has received two doses of Aimovig and has so far had no effect, either positive or negative. For most of October, she barely left home in San Rafael. The pain, nausea and fatigue left her almost in bed. Getting up just one day for an appointment with Riggins was a challenge. November was not much better.
"I do not remember the last time I felt good," Marshall said.

Her three teenagers all suffer from migraines, to varying degrees. What annoys Marshall more than anything else, she said, is that her own migraines leave her feeling ill-equipped to support her children.
Trying migraine medications can be a roller coaster of emotional and physical effects, she said. She thinks she started having migraines in her childhood, then they became serious in their thirties. Ten years ago, migraines became chronic and hit her almost every day. But the drugs she tried to treat often did not work at all and did not cause unbearable side effects.
That the Aimovig is not working for her yet is discouraging, but not surprising. She can never answer it. It may also take a few months for the drug to begin functioning – typical of other medications given to migraine patients. This means that she has to be patient a little longer.
In the meantime, she and her husband fought for her insurance company to pay for the therapy. She has been refused twice and, like Brook, the drug developer is paying Aimovig for the moment.
"Honestly, at this point, I'm trying to stay away from the whole insurance business," Marshall said. "I can not invest emotionally in all this nonsense because it's too exhausting. And I just do not have the energy. "
Erin Allday is a writer at the San Francisco Chronicle. Email: [email protected] Twitter: @erinallday
[ad_2]
Source link