[ad_1]

GlaxoSmithKline (GSK) and Medicines for Malaria Venture (MMV) announced that the US Food and Drug Administration (FDA) has approved, as part of a priority review, kinétra (tafenoquine) single dose for radical cure (prevention of relapse) of Plasmodium vivax malaria (P. vivax) in patients 16 years of age and older receiving appropriate antimalarial treatment for acute P. vivax infection.

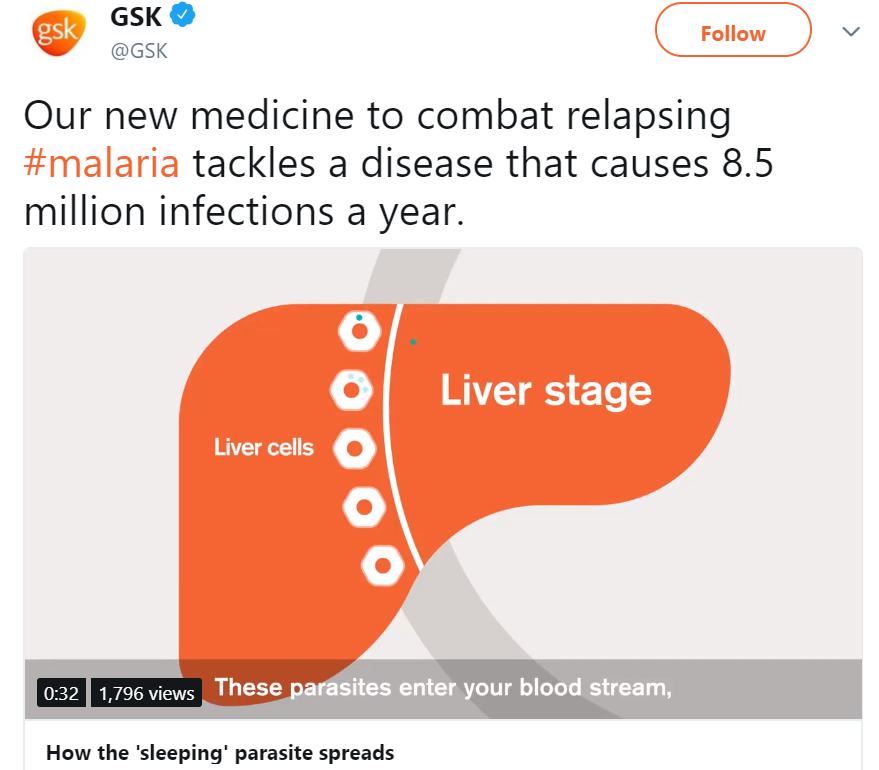
Krintafel is an 8-aminoquinoline derivative with activity against all life cycle stages of P. vivax , including hypnozoites
. Hal Barron, Scientific Director and President of Research and Development, GSK, said: "The approval of Krintafel, the first new treatment against Plasmodium vivax malaria for more than 60 years, is a milestone for people with Alzheimer's disease. of this type of recurrent malaria. In collaboration with our partner, Medicines for Malaria Venture, we believe that Krintafel will be an important medicine for malaria patients and will contribute to ongoing efforts to eradicate this disease. "
Dr. David Reddy, Managing Director of MMV, said," The approval of Krintafel by the US FDA is a milestone and a significant contribution to global efforts to eradicate malaria. has waited decades for a new drug to counter the relapse of P. vivax malaria.Today, we can say that the wait is over.In addition, as the first single dose for this indication, Krintafel will help improve patient compliance.We are proud to have worked side by side with GSK for more than a decade to reach this point.Our goal is now to work for the drug to reach vulnerable patients. who needed it the most. "
The approval was based on the efficacy and tolerability data of a global overall clinical development. radical healing program designed in agreement with the FDA. Thirteen studies in healthy volunteers and patients directly supported the program.
P. Vivax malaria has a significant impact on public health and the economy, mainly in South-East Asia, South-East Asia, Latin America and the Horn of Africa. ;Africa. The disease is estimated at about 8.5 million clinical infections each year. The clinical manifestations of P. vivax malaria include fever, chills, vomiting, malaise, headache and muscle aches and, in some cases, can lead to severe malaria and death.
Source link