[ad_1]
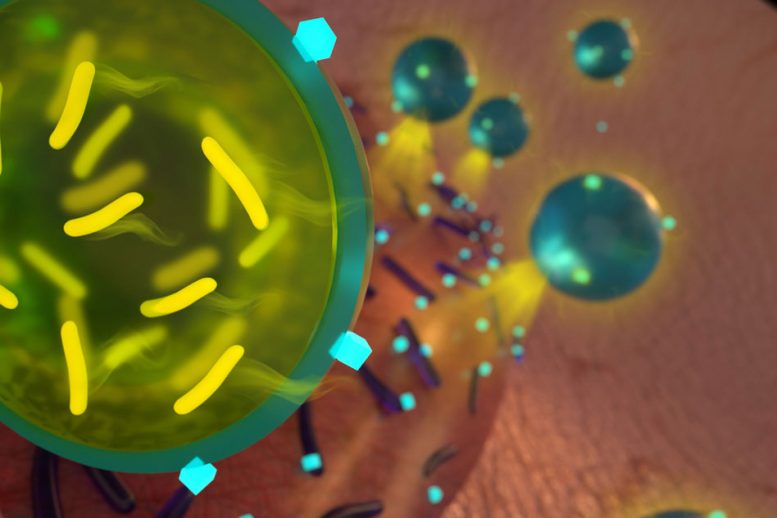
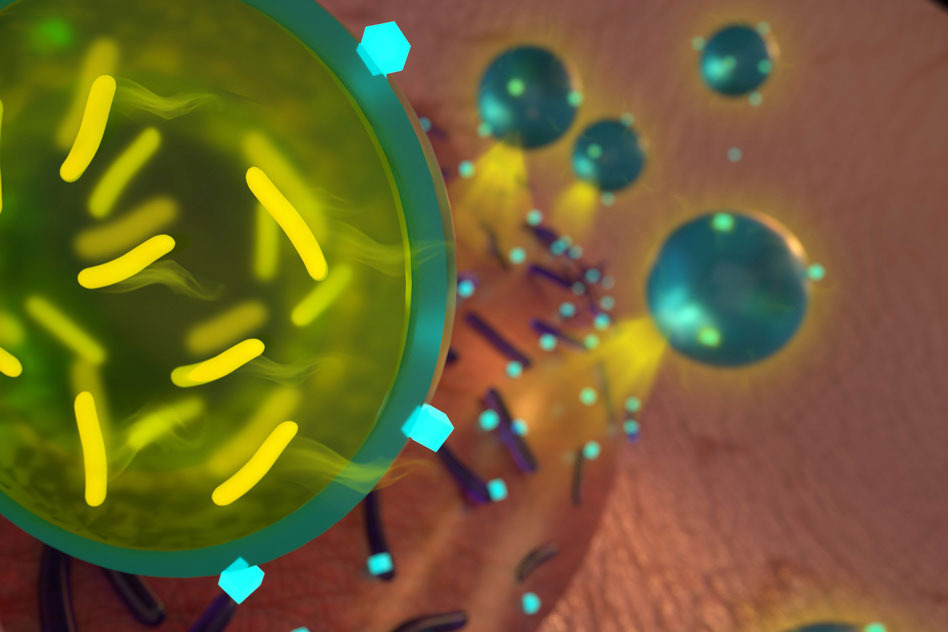
MIT's chemical engineers have come up with a way to encapsulate the probiotics so that they can be administered with antibiotics to eliminate several strains of bacteria. Image: Ryan Allen
In the fight against drug-resistant bacteria, MIT the researchers used beneficial bacteria called probiotics.
In a new study, researchers have shown that by using a combination of antibiotics and probiotics, they can eradicate two strains of drug-resistant bacteria that often infect wounds. To do this, they encapsulated the probiotic bacteria in a protective alginate shell, a biocompatible material preventing probiotics from being killed by the antibiotic.
"There are so many bacteria that are now resistant to antibiotics, which is a serious problem for human health. We believe that one way to treat them is by encapsulating a live probiotic and letting it do its job, "says Ana Jaklenec, a researcher at the Koch Institute for Cancer Research and a member of MIT, and one of the main authors of the study.
If future tests on animals and humans prove effective, the probiotic / antibiotic combination could be incorporated into wound dressings, where it could help heal infected chronic wounds, the researchers say.
Dr. Robert Langer, a professor at the David H. Koch Institute and a member of the Koch Institute, is also the lead author of this article, published in the Advanced Materials journal of 17 October. Zhihao Li, a former MIT visiting scientist, is the lead author of the study.
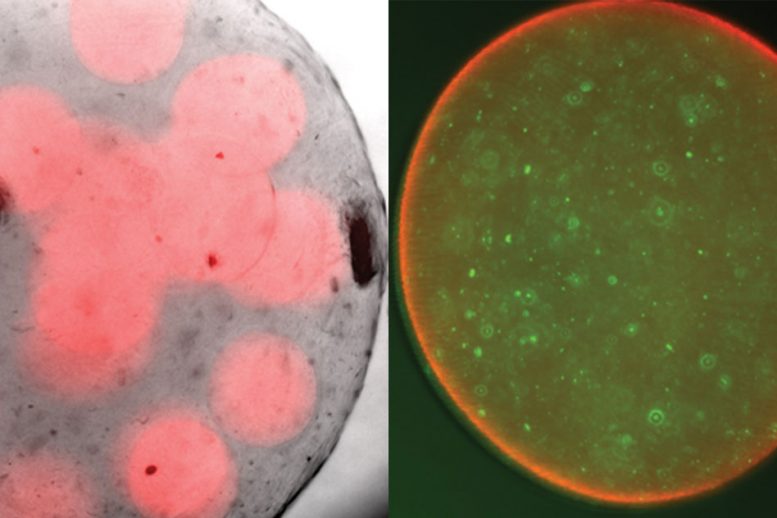
On the left, alginate spheres with probiotics encapsulated on the inside. On the right, a close-up of a single sphere, with probiotic bacteria marked in green. Courtesy of the researchers
Bacteria wars
The human body contains billions of bacterial cells, many of which are beneficial. In some cases, these bacteria help prevent infection by secreting antimicrobial peptides and other compounds that kill pathogenic strains of bacteria. Others outperform harmful strains by absorbing nutrients and other essential resources.
Scientists have already tested the idea of applying probiotics to chronic wounds, and they have had some success in studies on burn patients, says Li. However, probiotic strains generally can not fight off all the bacteria present in an infected wound. Combining these strains with traditional antibiotics would help kill more pathogenic bacteria, but the antibiotic could also kill probiotic bacteria.
The MIT team has devised a way to solve this problem by encapsulating probiotic bacteria so that they are not affected by the antibiotic. They chose alginate in part because it is already used in dressings for chronic wounds, where it helps to absorb secretions and keep the wound dry. In addition, researchers have also discovered that alginate is a component of biofilms that form clusters of bacteria to protect themselves from antibiotics.
"We looked at the molecular components of biofilms and found that for Pseudomonas infection, alginate is very important for its resistance to antibiotics," says Li. "However, no one has still used this ability to protect the good bacteria from antibiotics. "
For this study, researchers chose to encapsulate a commercially available type of probiotic called Bio-K +, consisting of three strains of Lactobacillus bacteria. These strains are known to kill methicillin-resistant Staphylococcus aureus (MRSA). The exact mechanism by which they do this is not known, but it is possible that the pathogens are sensitive to the lactic acid produced by the probiotics. Another possibility is that probiotics secrete antimicrobial peptides or other proteins that kill pathogens or disrupt their ability to form biofilms.
The researchers delivered the encapsulated probiotics together with an antibiotic called tobramycin, which they chose from among other antibiotics tested, as it effectively kills Pseudomonas aeruginosa, another strain commonly found in wound infections. . When MRSA and Pseudomonas aeruginosa grown in a laboratory capsule were exposed to the combination of encapsulated Bio-K + and tobramycin, all pathogenic bacteria were removed.
"It was a pretty drastic effect," says Jaklenec. "He completely eradicated the bacteria."
When they tried the same experiment with unencapsulated probiotics, they were killed by antibiotics, thus allowing the MRSA bacteria to survive.
"When we only used one component, antibiotics or probiotics, they could not eradicate all the pathogens. This is something that can be very important in clinical settings where you have wounds with different bacteria and antibiotics are not enough to kill all the bacteria, "says Li.
Better healing
The researchers believe that this approach could be used to develop new types of bandages or other wound dressings containing antibiotics and probiotics encapsulated in alginate. Before this can happen, they plan to further test the approach in animals and possibly in humans.
"The good thing about alginate is that it's FDA approved and the probiotic we use is also approved," says Li. "I think probiotics can revolutionize wound treatment in the future." . Through our work, we have expanded the application possibilities of probiotics. "
In a study published in 2016, researchers demonstrated that covering probiotics with layers of alginate and another polysaccharide called chitosan could protect them from degradation in the gastrointestinal tract. This could help researchers develop ways to treat diseases or improve digestion with probiotics administered orally. Another potential application is to use these probiotics to replenish the intestinal microbiome after antibiotic treatment, which can eliminate beneficial bacteria while eliminating infection.
Li's work on this project was funded by the Swiss Janggen-Poehn Foundation and Beatrice Beck-Schimmer and Hans-Ruedi Gonzenbach.
Publication: Zhihao Li, et al., "Encodulation of probiotics by a biofilm for the treatment of complex infections," Advanced Materials, 2018; doi: 10.1002 / adma.201803925
[ad_2]
Source link