[ad_1]
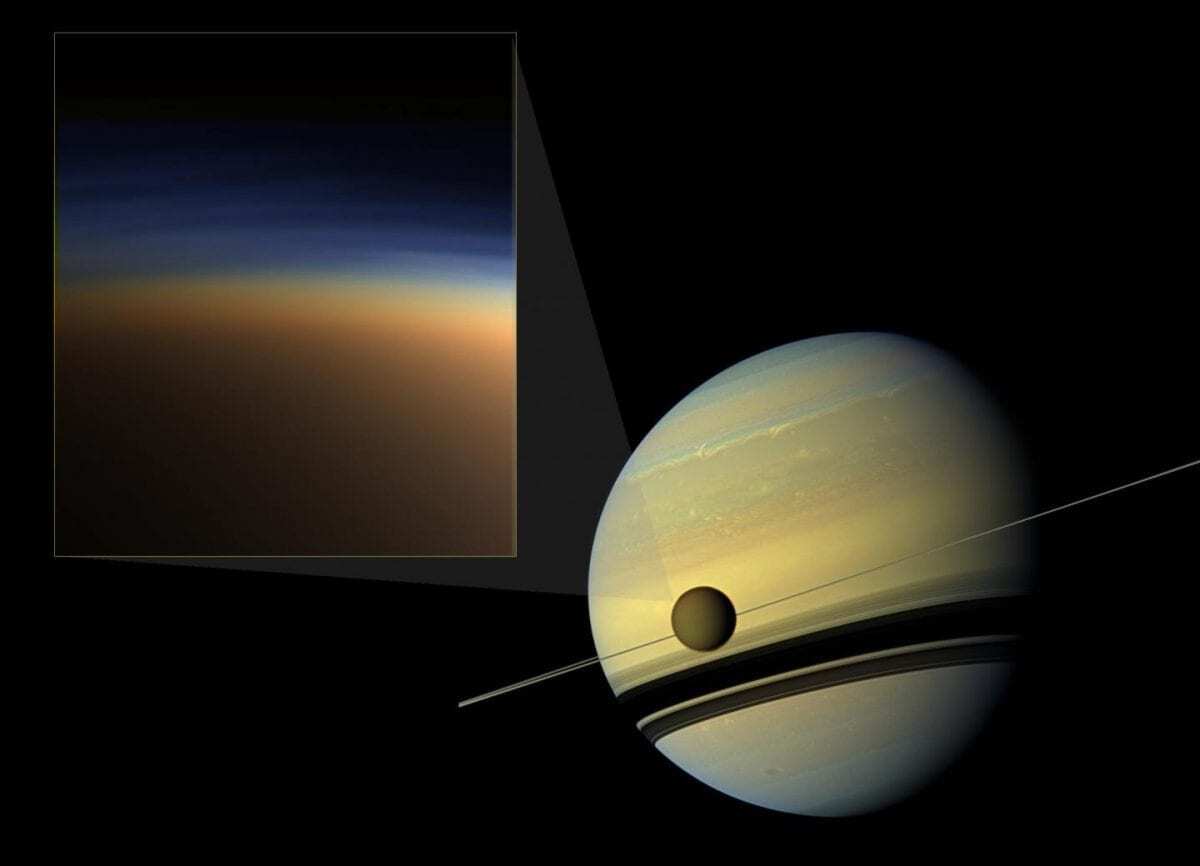
Saturn's largest moon, Titan, is unique among all the moons of our solar system for its dense, nitrogen-rich atmosphere, which also contains hydrocarbons and other compounds. The history of the formation of this rich mixture of chemicals has been at the origin of a scientific debate. .
At present, a research collaboration involving scientists from the Chemical Sciences Division of the Lawrence Berkeley National Laboratory (Berkeley Laboratory) of the Department of Energy has focused on a low-temperature chemical mechanism that could have led to the formation of multi-cycle molecules – the precursors of a more complex chemistry is now found in the orange-brown haze layer of the moon.
The study, co-directed by Ralf Kaiser of the University of Hawaii at Manoa and published in the October 8 edition of the journal Nature Astronomy, goes against the theories that high-temperature reaction mechanisms are required to produce the chemical composition observed by satellite missions in Titan's atmosphere.
The team also included other researchers from the Berkeley Lab, the University of Hawaii at Manoa, the University of Samara in Russia and the International University of Florida. The team used vacuum ultraviolet light experiments at Berkeley Lab's Advanced Light Source (ALS), as well as computer simulations and modeling work to demonstrate the chemical reactions that contribute to Titan's modern atmospheric chemistry.
"We are providing here evidence of a low-temperature reaction process that people have not thought of," said Musahid Ahmed, a scientist at the Berkeley Lab's Chemical Sciences Division and co-head of the study at the University of California. ALS. "This creates a missing link in Titan's chemistry."
Titan could give clues about the development of complex chemistry on other moons and planets, including the Earth, he explained. "People use Titan to think of a" pre-biotic "Earth, while nitrogen was no longer present in the Earth's atmosphere at first."
Benzene, a single hydrocarbon having a six-carbon ring molecular structure, has been detected on Titan and is thought to be a building block for larger hydrocarbon molecules with two- and three-ring structures that, in turn, have formed other hydrocarbons and aerosol particles that now make up Titan 's atmosphere. These multi-ring hydrocarbon molecules are known as polycyclic aromatic hydrocarbons (PAHs).
In the latest study, the researchers mixed two gases – a short-lived two-ring PAH called a naphthyl radical (C10H7) and a hydrocarbon called vinylacetylene (C4H4) – during ALS and produced three-cycle PAHs. The two chemicals used to drive the reaction are thought to exist on Titan based on what is known about the chemical composition of its atmosphere.
The ALS experiments threw the final products of the reactions into a small reaction chamber. The researchers used a detector called a reflectron time-of-flight mass spectrometer to measure the mass of molecular fragments produced during the reaction of the two gases. These measurements provided details on the chemistry of the three-ring PAHs (phenanthrene and anthracene).
While the ALS experiments used a chemical reactor to simulate the chemical reaction and a vacuum ultraviolet light beam to detect the reaction products, calculations and complementary simulations showed that the chemicals formed in the ALS experiments did not require high temperatures.
PAHs, like the chemicals studied at ALS, have properties that make them particularly difficult to identify in remote areas, Kaiser said. "In fact, not one single PAH has been detected in the gaseous phase of the interstellar medium," which is the material that fills the space between the stars.
He added, "Our study demonstrates that PAHs are more widespread than expected because they do not require the high temperatures present around carbon stars. This mechanism we have explored should be versatile and lead to the formation of even more complex PAHs. "
And since PAHs are considered precursors to the formation of molecular clouds – the so-called "molecular factories" of more complex organic molecules that may include the precursors of life as we know them – "This could open theories and new models on how carbon The matter contained in the deep space and in the rich atmosphere of the planets and their moons in our solar system evolves and originates, "he said.
Alexander M. Mebel, professor of chemistry at Florida International University and co-head of the study, performed calculations that showed how reagents can naturally collect and form new compounds at very low temperatures.
"Our calculations revealed the reaction mechanism," Mebel said. "We have shown that you do not need any energy to drive the reaction of naphthyl and vinylacetylene, so the reaction must be effective even under low-temperature, low-pressure atmospheric conditions on Titan."
Detailed modeling of the reactor cell where the gases were mixed was one of the keys to the study.
Mebel noted that the modeling of the energies and the simulation of the gas flow dynamics involved in the reactor made it possible to follow the evolution of the reaction in the reactor and allowed the researchers to closely link the theoretical results. to experimental observations.
The modeling work, which predicted the chemicals produced in the reactions based on the initial gases as well as the temperature and pressure of the heated chamber where the gases were mixed and struck with the ultraviolet beam under vacuum, was led by the research team of Samara University. .
"This model verification, comparing it to experiments, can also be useful in predicting how the reaction would unfold under different conditions – from Titan's atmosphere to burning flames on Earth."
According to Dr. Kaiser, one of the goals of current research is to explain in detail how carbon-containing compounds and structures with similar DNA and RNA structures can develop even in extreme environments.
DOE / LAWRENCE BERKELEY NATIONAL LABORATORY
Header Image – Titan's atmospheric haze, Saturn's largest moon (pictured here along the mid-section of Saturn), is captured in this natural-colored image (left-hand framed). A new study, which included experiments conducted in the Berkeley Lab Advanced Light Source, provided new clues about the chemical steps that could have produced this veil. Credit: NASA Jet Propulsion Laboratory, Institute of Space Science, Caltech
 |
 |
[ad_2]
Source link