[ad_1]
MONDAY, Sept. 24, 2018 (HealthDay News) – A paraplegic man has regained the ability to move his legs and walk with the help of an implanted electrode that stimulates his spinal cord, researchers at Mayo Clinic say.
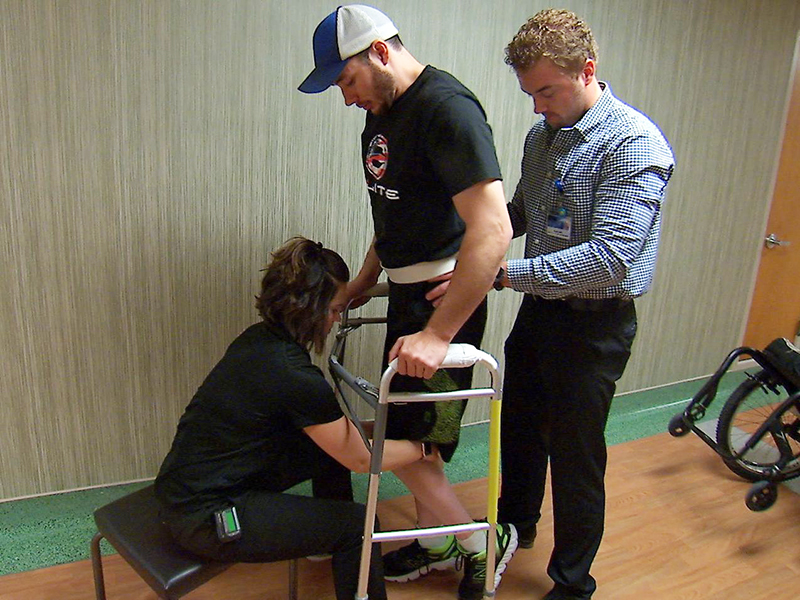
Surgeons implanted the electrode below the 29-year-old Jorn Chinnock spinal cord injury level. A snowmobile accident in 2013 caused a total loss of motor control and mid-back feel.
But after the new therapy, he "was able to regain the voluntary control of the movement in his legs," said Deputy Principal Investigator, Dr. Kendall Lee, neurosurgeon and director of Neural Engineering Labs at Mayo Clinic in Rochester , Minnesota. The "mind or thoughts of Chinnock were able to drive the movement in the legs".
Similar results were also reported Monday for patients who received the same type of treatment in a study conducted at the University of Louisville.
Describing the case of Chinnock, the researchers said that he can now walk the length of a football field, about 111 meters.
"We managed to convince him to run independently and take his own action," Lee said. "The number of steps that he was able to cross was considerable."
New perspectives on the spinal cord
Researchers do not know why this electrical stimulation allows the brain to regain control of the legs, Lee said.
He noted that the electrode is placed "well below the level of the injury," stimulating the nerve tissue that is still connected to the leg muscles.
According to Kristin Zhao, co-principal investigator and director of the Mayo Clinic's Assistance and Restoration Technology Laboratory, it is possible that, despite the injury, there are residual intact nerve fibers capable of transmitting cerebral signals to the legs. .
If this is the case, the brain is likely sending signals to re-stimulated nerves further into the spinal cord that are specifically related to walking, said Dr. Brian Kopell, neurosurgeon and director of the Mount Health System Neuromodulation Center. Sinai. York City.
"We are beginning to understand that there are specific wired circuits related to walking in the spinal cord itself," said Kopell, who was not involved in the study. "The brain works in conjunction with these locomotor sectors in the spinal cord to create the behavior we know as walking."
How it works
This study began in 2016, with Chinnock receiving its electrode implant after 22 weeks of physical therapy.
It is found in the epidural space that covers the spinal cord, said Lee. He is connected to a pulse generator implanted just under the skin of his abdomen.
Researchers can wirelessly program the pulse generator to provide specific electrical stimulation to the spinal cord, Lee said.
After her recovery, Chinnock had 43 weeks of intense physical therapy involving 113 visits to the Mayo Clinic, Zhao said.
He eventually regained the ability to walk on the floor using a wheeled walker and on a treadmill with his arms on support bars to help balance.
At the end of the study period, Chinnock learned to use his entire body to transfer weight, maintain balance and propel himself forward, according to the researchers.
But his legs only move when the pulse generator is activated, Lee said.
"The stimulation must absolutely be activated," Lee said. "We have found that you need to provide a very specific kind of stimulation, random stimulation does not work."
Chinnock still can not feel anything under the site of his spinal cord injury, Lee said.
He can not still walk independently outside the lab, but does regular exercise at home when he is standing or sitting, Zhao said.
Total independence from the lens
Chinnock said the implant has also helped in one of his favorite hobbies, hunting bow.
"My sitting balance and my business have improved a lot as I can better shoot my bow because I can hold – have more support on the trunk," he said in a video released by Mayo.
Chinnock said his goal was to become "completely independent – to be where I needed a walker, but I did not need anyone to help me." I mean, it's a goal, but the main goal is to not need anything. "
The electric stimulator it has is the one designed for nerve pain. The research team has received approval from the US Food and Drug Administration to use this new method.
Researchers are now considering taking a step back and reorganizing the device to specifically target paralysis, Lee said.
They also plan further studies to determine what is happening in the brain and spinal cord, allowing a patient to regain control of his legs, Zhao said.
The latest report on this study appears in the journal Medicine of nature.
In a similar separate study published this week in the New England Journal of MedicineResearchers at the University of Louisville reported that two of the four paralyzed patients were able to walk again after receiving implanted stimulation and intense physical therapy.
Susan Harkema, a professor of neurological surgery who was part of this research, described the result as "phenomenal" in an interview with CNN.
"This new knowledge gives us the tools to develop new strategies and recovery tools for people with chronic spinal cord injuries," she said.
Harkema and colleagues implanted epidural stimulators in 14 paralyzed patients over the years. Thanks to the devices, all 14 were able to move and had better intestinal and bladder function, she said.
"This should change the way we think about people with paralysis," Harkema said.
More information
Columbia University has more on neuromodulation.
SOURCES: Kendall Lee, MD, Ph.D., neurosurgeon and director, Neural Engineering Laboratories, Mayo Clinic, Rochester, Minn .; Kristin Zhao, Ph.D., Director, Assistance and Restoration Technology Laboratory, Mayo Clinic; Brian Kopell, MD, neurosurgeon and director, Neuromodulation Center, Mount Sinai Health System, New York City; Medicine of natureSeptember 24, 2018; CNN
[ad_2]Source link