[ad_1]
The US pharmaceutical company ImmusanT, after tests conducted around the world, is about to begin clinical trials of a new celiac disease vaccine at the Royal Melbourne Hospital's clinical trials center in Melbourne. Trials will be held in Adelaide, Brisbane, Perth, Mackay and Sunshine Coast, the sources said.

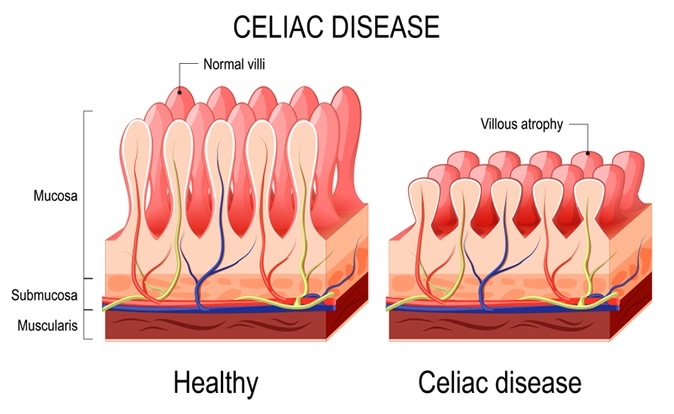
Celiac disease – normal villi and villous atrophy. Image credit: Designua / Shutterstock
The injectable vaccine was developed about eight years ago in Melbourne and has proven safe in early trials with a small number of participants. The disease is basically an exaggerated immune response from the intestine to food allergens such as gluten. Among patients with celiac disease, these food allergens cause severe allergic reactions leading to gastrointestinal disorders. There are currently about 160,000 Australians with celiac disease who are forced to go on a diet totally devoid of gluten to prevent debilitating symptoms. This means that about one in 70 Australians are living with celiac disease, say the researchers who developed the vaccine. Gluten is a protein component found in some cereals such as rye, wheat and barley.
This new vaccine called Nexvax2 can reprogram the immune response to gluten. This prevents and alleviates symptoms. It was developed at the Walter and Eliza Hall Medical Research Institute (WEHI). The team discovered three peptides in gluten that usually trigger the symptoms in 90% of cases and created the vaccine against these peptides.
For this latest Phase 2 clinical trial, approximately 50 patients from Australia, New Zealand and the United States would be recruited. They would receive injections of the vaccine in the trial or placebo twice a week for four weeks. Patients would not know which one (vaccine or placebo) they received. Subsequently, test participants would be faced with three dietary problems to see if they were receiving symptomatic relief. The protective effects of the lining of the intestinal wall would be tested by performing a biopsy of the internal walls of the intestine.
The team explains that the vaccine can prevent excessive sensitivity of immune cells from the intestines in sensitive people. These T cells do not therefore go into "attack mode" in the face of gluten. According to vaccine designers, it may be beneficial for 90% of patients with the HLA-DQ2 genetic form.
Vaccine developers led by Dr. Jason Tye-Din, WEHI and Royal Melbourne Hospital, explain that there have been national and international phase 1 trials in which they have examined the highest possible dose of the vaccine. and have always found it safe and well tolerated. Dr. Tys-Din said, "This has also shown a predicted biological effect on the immune system in patients with celiac disease. The Phase 2 trials are based on previous study data and it is good that Australia still plays a central role in this work. "
"Effective treatment that would restore normal gluten tolerance would revolutionize the management of celiac disease," he said.
A list of hospitals where the trial would be conducted can be found at www.coeliac.org.au
[ad_2]
Source link