[ad_1]
The US National Institute of Heart, Lung and Blood (NHLBI) has suspended a clinical trial of stem cells to treat heart failure after being questioned about the validity of the science that underwent it. -tend.
The institute took the decision "for the sake of caution, to ensure that the study continues to meet the highest standards of participant safety and scientific integrity," the statement said. in a statement published on October 29.
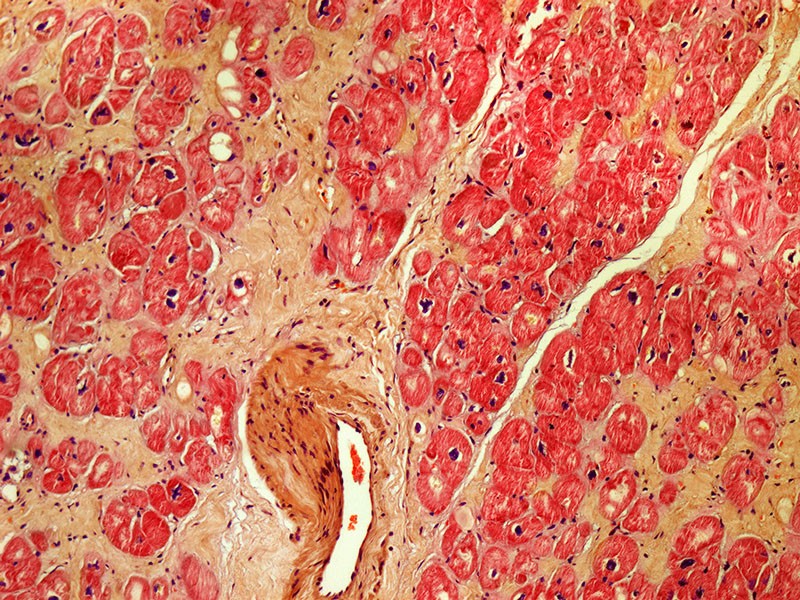
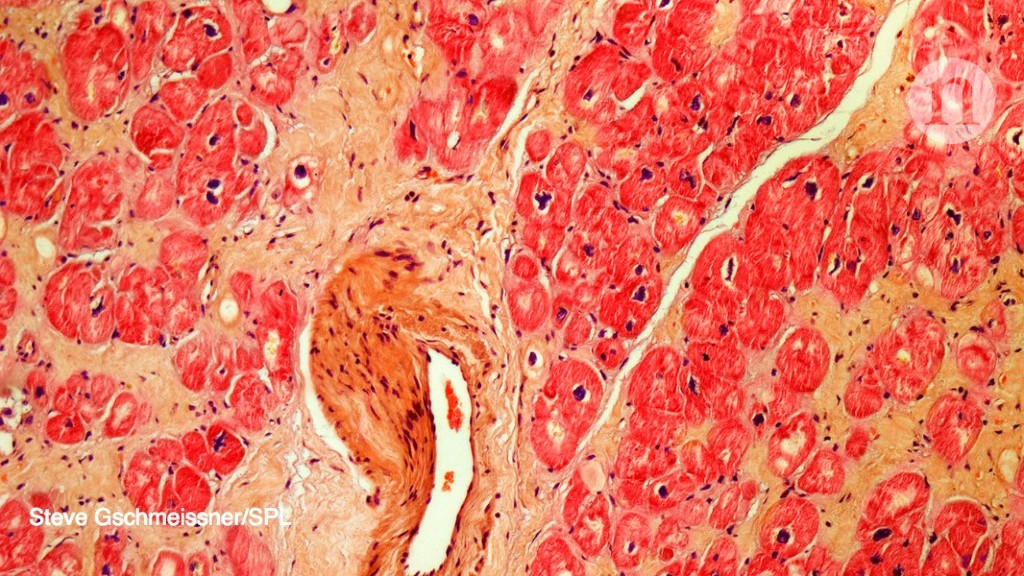
The study was based on the research of cardiologist Piero Anversa of Harvard Medical School and Brigham and Women's Hospital in Boston. In 2002, he suggested that a type of stem cell in the heart, called c-kit cells, could regenerate the heart muscle.1. The NHLBI study has given people with chronic heart failure infusions of c-kit cells or combinations of c-kit cells and bone marrow stem cells. It was launched in 2015 with the goal of enrolling 144 participants. so far, 125 patients have enrolled.
The work of Antwerpa aroused doubt in the early 2000s, after other researchers failed to replicate its findings and wondered if there were cardiac stem cells.2,3,4.
Antwerpa closed its laboratory in 2015 and left Harvard. In 2017, Partners HealthCare System – which manages Brigham and Women's Hospital – donated US $ 10 million to the US government in settling charges that the Antwerp team allegedly submitted fraudulent data to to obtain federal funds.
Earlier this month, the hospital and Harvard asked newspaper publishers to remove 31 items from Antwerpa after their investigations revealed that they contained manipulated or manufactured data.
Antwerpa could not be contacted immediately for comment.
The NHLBI states that the decision to discontinue the study was due to concerns about Antwerpa animal studies and not to data generated by the trial itself. The agency said that she did not believe that the trial had compromised patient safety.
Sign up for the everyday Nature Briefing email
Stay informed about what matters in science and why, chosen by hand Nature and other publications worldwide.
S & # 39; register
Source link