
[ad_1]
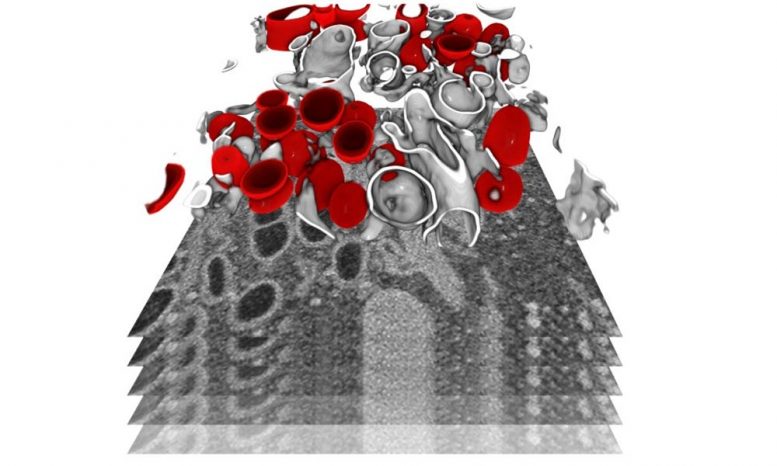
The infected cells were imaged by focused ion beam scanning electron microscopy, a powerful technique to reveal the organization of a cell at the subcellular level in 3D. In this image, a subvolume of a cell has been segmented to show membrane-bound organelles (in gray) and double membrane vesicles (in red) – a virus-specific compartment where the viral genome is replicated in large quantities. Credit: Julian Hennies / EMBL
Learn how SARS-CoV-2 Highjack host cell machines will help develop therapeutic strategies.
As the global coronavirus pandemic continues, scientists are not only trying to find vaccines and drugs to fight it, but also continually learn more about the virus itself. “Now we can expect the coronavirus to become seasonal,” says Ralf Bartenschlager, professor in the Department of Infectious Diseases, Molecular Virology, at the University of Heidelberg. “It is therefore urgent to develop and implement both prophylactic and therapeutic strategies against this virus.” In a new study, Bartenschlager, assisted by the Schwab team at EMBL Heidelberg and using EMBL’s electron microscopy powerhouse, performed detailed imaging analysis to determine how the virus reprograms infected cells.
Cells infected with SARS-CoV-2 die fairly quickly, in just 24 to 48 hours. This indicates that the virus harms the human cell in such a way that it is rewired and essentially forced into producing viral progeny. The main objective of the project was therefore to identify the morphological changes within a cell which are inherent in this reprogramming. “Developing drugs which suppress viral replication and therefore the consequences of infection, as well as cell death induced by the virus, is essential to have a better understanding of the biological mechanisms driving the replication cycle of the virus”, explains Bartenschlager. The team used EMBL’s imaging facilities and state-of-the-art imaging techniques to determine the 3D architecture of cells infected with SARS-CoV-2, as well as alterations in cell architecture caused by the virus.
The team was able to create 3D reconstructions of whole cells and their subcellular compartments. “We provide essential information on the structural changes induced by viruses in the human cells studied,” explains Ralf Bartenschlager. The images revealed an obvious and massive change in the endomembrane systems of infected cells – a system that allows the cell to define different compartments and sites. The virus induces membrane changes so that it can produce its own replicating organelles. These are mini replication compartments where the viral genome is enormously amplified. To do this, the virus needs membrane surfaces. These are created by harnessing a cell membrane system and creating an organelle, which has a very distinct appearance. Scientists describe it as a massive accumulation of bubbles: two membrane layers forming a large balloon. In these balloons – which form a highly protected compartment – the viral genomes are multiplied and released to be incorporated into new viral particles.
This striking change is not visible in the cells until a few hours after infection. “We saw how and where the virus replicated in the cell, and how it hijacked its host machinery to be released after multiplication,” Schwab explains. Until now, little was known about the origin and development of the effects of SARS-CoV-2 on the human body. This includes a lack of knowledge about the mechanism by which infection leads to the death of infected cells. Having this information now will promote the development of therapies that reduce virus replication and, therefore, the severity of the disease.
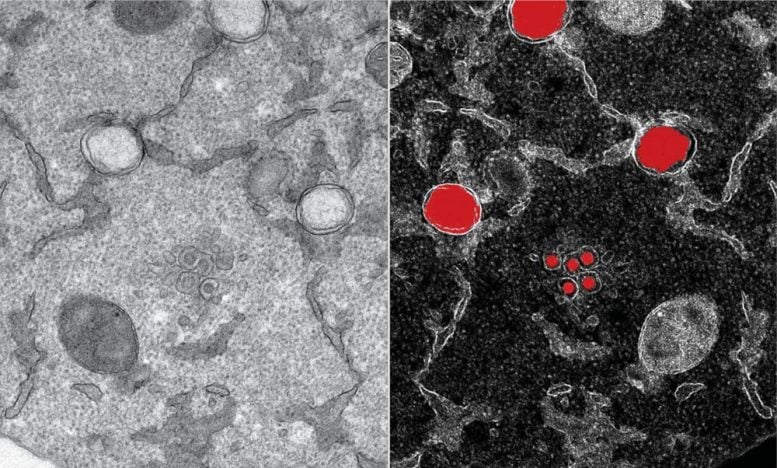
Part of an infected cell is observed by transmission electron microscopy, in which structures specific to SARS-CoV-2 (in red, from the mirror image to the right) can be detected as early as six hours after the infection. The virus genome is replicated in large number of copies in two membrane layers forming a large balloon (large structures in red), which forms a highly shielded compartment. New virions (small structures in red) are formed by budding at the interface of the endoplasmic reticulum and the Golgi apparatus. Credits: Yannick Schwab / EMBL
The team ensured that the information collected and, in particular, the unprecedented repository of 3D structural information on virus-induced substructures could be used by anyone. “I think we are setting a precedent that we share all the data that we have produced with the scientific community. It represents an impressive resource for the community, ”says Yannick Schwab. “In this way, we can support the global effort to study how SARS-CoV-2 interacts with its host.” The team hopes the information gathered will aid in the development of antiviral drugs.
The team managed to produce the study in an incredibly short time, despite the difficult circumstances. “Half the world – and of course Heidelberg too – were in full lockdown and we had to improvise almost daily to adjust to the situation. Whether at EMBL or from home, everyone was deeply involved and generously volunteered their time and in-depth knowledge, ”says Schwab. “The speed at which we worked and the amount of data produced is remarkable.”
Reference: “Integrative imaging reveals SARS-CoV-2-induced remodeling of subcellular morphologies” by Mirko Cortese, Ji-Young Lee, Berati Cerikan, Christopher J. Neufeldt, Viola MJOorschot, Sebastian Köhrer, Julian Hennies, Nicole L .Schieber, Paolo Ronchi, Giulia Mizzon, Inés Romero Brey, Rachel Santarella-Mellwig, Martin Schorb, Mandy Boermel, Karel Mocaer, Marianne S. Beckwith, Rachel M. Templin, Viktoriia Gross, Constantin Pape, Christian Tischer, Jamie Frankish, Natalie K. Horvat, Vibor Laketa, Megan Stanifer, Steeve Boulant, Alessia Ruggieri, Laurent Chatel-Chaix, Yannick Schwab and Ralf Bartenschlager, November 17, 2020, Cell host and microbe.
DOI: 10.1016 / j.chom.2020.11.003
[ad_2]
Source link