[ad_1]
An effective immune response to the general signs of infection, regulated by the immune system's branch called innate immunity, is essential for removing unwanted bacteria. Such a response should then end when the infection is too heavy and blocks any unwanted inflammatory response. The processes that determine the effectiveness or dysfunction of inflammation are of considerable therapeutic interest, given the lack of strategies available to target harmful inflammation while preserving the beneficial defenses of the disease. host. Efforts to understand how immune cells respond to inflammation have in turn drawn attention to the processes of immune regulation. These include the processes involved in detecting the damage associated with the infection1as well as those needed to recognize other changes related to infection, such as2 or oxygen levels3,4. Write in Nature, Solis et al.5 reveal that the mechanical signals generated in the mouse lung are detected by the immune cells and are essential regulators of the immune response.
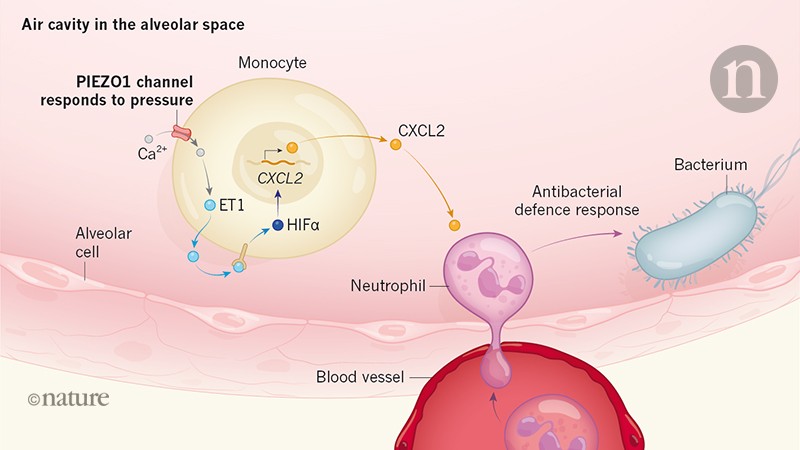
Myeloid cells of the immune system – a group comprising macrophages and monocytes – are exposed to various physical forces, for example, those encountered when penetrating blood vessels into tissues.6. The cycles of mechanical force occur in organs such as the lungs, in which tissues are compressed during breathing7. These forces are themselves subject to change in the pathological state; for example, when the tissue swells during an inflammatory response. Solis and his collaborators report that macrophages and monocytes can respond to mechanical signals perceived via a mechanosensory ion channel called PIEZO1 located on the surface of their cell.
To understand whether the exposure of myeloid cells to mechanical forces could directly regulate the function of immune cells, the authors generated mice lacking PIEZO1 in myeloid cells. Using a in vitro system, the authors subjected immune cells to pressure change cycles mimicking those encountered in the lung, called cyclic hydrostatic pressure. The authors compared macrophages and wild-type monocytes deficient in PIEZO1, which revealed that cyclic hydrostatic pressure induces a pro-inflammatory gene expression profile in PIEZO1-dependent wild-type cells. This expression profile included genes controlled by the HIF1α transcription factor protein, a key regulator of gene expression required for the functioning and survival of myeloid cells.8–ten. Interestingly, this pro-inflammatory gene expression response was not affected by the magnitude of the pressure encountered.
To understand the mechanisms at the origin of this transcriptional response, the authors studied HIF1α-deficient macrophages. They discovered that cells were unable to develop a pro-inflammatory gene expression response to cyclic hydrostatic pressure. The authors reveal that subjecting wild type cells to this type of pressure in the in vitro The system causes an influx of calcium ions into the cells via the PIEZO1 channel, resulting in an accumulation of HIF1α (Fig. 1). This increase in HIF1Î ± induced by PIEZO1 required the production of the hormone endothelin 1, which acts in a signaling pathway that stabilizes HIF1Î ± in cells.11,12. Endothelin 1 is secreted by cells and acts by binding to its receptor, either on the cell that secreted it, or on a neighboring cell.
To test the role of PIEZO1 mediated signaling in host defenses, Solis and his colleagues used a mouse model of pneumonia in which the infection is caused by the bacteria. Pseudomonas aeruginosa. Compared to wild-type mice, animals modified by lack of PIEZO1 in myeloid cells had fewer immune cells called neutrophils in lung tissue and lower levels of pro-inflammatory immune signaling molecules in the lungs, such as lymphatic cells. endothelin 1. had lower levels of the CXCL2 protein, which attracts neutrophils. These mice had higher levels of bacteria in their lungs and greater bacterial spread in the liver compared to wild-type mice.
The authors report that endothelin 1 production was not affected if PIEZO1 was depleted in macrophages of mice found in a pulmonary structure called alveolus, or if the ion channel was depleted in dendritic cells, which are another type of pulmonary immune cells. However, decreased monocytes caused a reduction in endothelin 1 levels, implicating cells as the source of this hormone. The authors confirmed that PIEZO1-dependent endothelin-1 production had a critical role in defense against infection by showing that endothelin-1 administration to mice lacking PIEZO1 in myeloid cells reduced the bacterial load. in relation to the bacterial load in these animals. The work of Solis and his colleagues is consistent with a model in which PIEZO1-mediated mechanosensitivity by monocytes in the lungs activates these cells to produce endothelin-1, resulting in an increase in the level of HIF1α and a gene pro- inflammatory. Expression profile. In turn, this leads to the recruitment of neutrophils, which help eliminate unwanted bacteria.
These observations raise key questions about the broader significance of PIEZO1 signaling in other diseases associated with impaired lung mechanics, such as pulmonary fibrosis. This condition is characterized by high levels of immune cells in the lungs, a reduction in lung elasticity and a restricted airflow. Solis and colleagues report that mice lacking PIEZO1 in myeloid cells are protected against lung injury in a murine model of pulmonary fibrosis, suggesting that the function of immune cells regulated by PIEZO1 may play a role in human disease. This should be a focus area during the continuation of these studies.
Understanding how signals are integrated to induce an effective immune response will require a deeper understanding than we have now. This is relevant in this case because the immune cells move between different compartments of the lung and are thus exposed to a range of environmental signals. Although PIEZO1 may promote a pro-inflammatory response that promotes the elimination of unwanted bacteria, the loss of this ion channel may also be beneficial, as it may protect from damaging inflammation associated with the mouse model of pulmonary fibrosis . Dissection of regulatory steps that maintain a balanced and effective immune response will be required to explore therapeutic avenues for targeting mechanosensory pathways during pulmonary inflammation.
[ad_2]
Source link