
[ad_1]
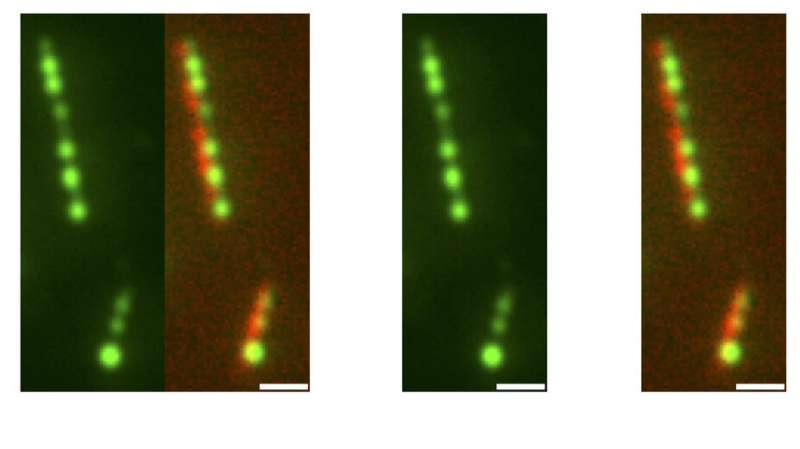
Researchers from the laboratories of Princeton University scientists Joshua Shaevitz, Howard Stone and Sabine Petry have found that surface tension causes the liquid-like protein TPX2 to form blood cells that nucleate the formation of branched microtubules during division cellular. The article detailing these findings appeared in the January 28 issue of the newspaper Physics of nature. Here, TPX2 (green) beads on microtubules (red) in the micrographs, with a one micron scale. Credit: Sagar U. Setru, Bernardo Gouveia, Raymundo Alfaro-Aco, Joshua W. Shaevitz, Howard A. Stone and Sabine Petry
As any cook knows, some liquids mix well, but others don’t. For example, when a tablespoon of vinegar is poured into water, a brief agitation is enough to mix the two liquids well. However, a tablespoon of oil poured into water will melt into droplets that no amount of stirring can dissolve. The physics of mixing liquids is not limited to mixing bowls; it also affects the behavior of things inside cells. It has been known for several years that certain proteins behave like liquids and that certain liquid-type proteins do not mix. However, very little is known about the behavior of these liquid-like proteins on cell surfaces.
“The separation between two liquids that don’t mix, like oil and water, is known as ‘liquid-liquid phase separation,’ and it’s central to how many proteins work,” said Sagar Setru, PhD in 2021. graduate who worked with Sabine Petry, professor of molecular biology, and Joshua Shaevitz, professor of physics and the Lewis-Sigler Institute for Integrative Genomics.
These proteins do not dissolve inside the cell. Instead, they condense with themselves or with a limited number of other proteins, allowing cells to compartmentalize certain biochemical activities without having to wrap them in membrane-bound spaces.
“In molecular biology, the study of proteins that form condensed phases with liquid-like properties is a rapidly growing field,” said Bernardo Gouveia, graduate student in chemical and biological engineering, working with Howard Stone, the Donald R Dixon ’69 and Elizabeth W. Dixon Professor of Mechanical and Aerospace Engineering and Department Head. Setru and Gouveia collaborated as co-first authors on an effort to better understand one of these proteins.
“We were curious about the behavior of the liquid-type TPX2 protein. What makes this protein special is that it does not form liquid droplets in the cytoplasm as had been observed previously, but rather appears to undergo phase separation on biological polymers called microtubules, “Setru says.” TPX2 is needed to create branched networks of microtubules, which is crucial for cell division. TPX2 is also overexpressed in some cancers, so understanding its behavior may be of medical importance. “
Individual microtubules are linear rod-shaped filaments. During cell division, new microtubules form on the sides of existing microtubules to create a branched network. The growth sites of new microtubules are marked by condensed TPX2 globules. These TPX2 globules recruit other proteins necessary for the growth of microtubules.
The researchers were curious about how TPX2 blood cells form on a microtubule. To find out, they decided to try and observe the process in action. First, they modified the microtubules and TPX2 so that each one glows with a different fluorescent color. Then they placed the microtubules on a microscope slide, added TPX2, and then watched what was going to happen. They also made observations at very high spatial resolution using a powerful imaging approach called atomic force microscopy.
“We found that TPX2 first covers the entire microtubule and then breaks down into droplets that are evenly spaced, much like morning dew covers a spider’s web and breaks down into droplets,” Gouveia said.
Setru, Gouveia, and colleagues found that this happens because of what physicists call Rayleigh-Plateau instability. Although non-physicists may not recognize the name, they will already be familiar with the phenomenon, which is why a stream of water falling from a faucet breaks into droplets, and why an even layer of water on a Strand of spider web blends into separate beads.
“It’s surprising to find such everyday physics in the nanoscale world of molecular biology,” Gouveia said.
Extending their study, the researchers found that the spacing and size of TPX2 blood cells on a microtubule is determined by the thickness of the initial TPX2 coating, that is, the amount of TPX2 present. This may explain why the branching of microtubules is impaired in cancer cells that overexpress TPX2.
“We used simulations to show that these droplets are a more efficient way of branching than just having a uniform coating or binding of the protein all along the microtubule,” Setru said.
“The fact that the physics of droplet formation, so clearly visible to the naked eye, has a role to play at the micrometer scale, helps to establish the growing interface (no pun intended) between the physics of soft matter and biology, ”said Rohit Pappu, the Edwin H. Murty professor of engineering at Washington University in St. Louis, who was not involved in the study.
“The underlying theory is likely to be applicable to an assortment of interfaces between liquid condensates and cell surfaces,” Pappu adds. “I suspect we’ll come back to this job over and over again.”
Researchers Uncover Secrets of Cell Division and Define Role of High Proteins in Cancer
Sagar U. Setru et al, Hydrodynamic instability leads to formation of protein droplets on microtubules to nucleate branches, Physics of nature (2021). DOI: 10.1038 / s41567-020-01141-8
Provided by Princeton University
Quote: Dewdrops on Spider Web Reveal Physics Behind Cellular Structures (2021, January 29) Retrieved January 30, 2021 from https://phys.org/news/2021-01-dewdrops-spiderweb-reveal- physics-cell.html
This document is subject to copyright. Other than fair use for study or private research, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link