[ad_1]
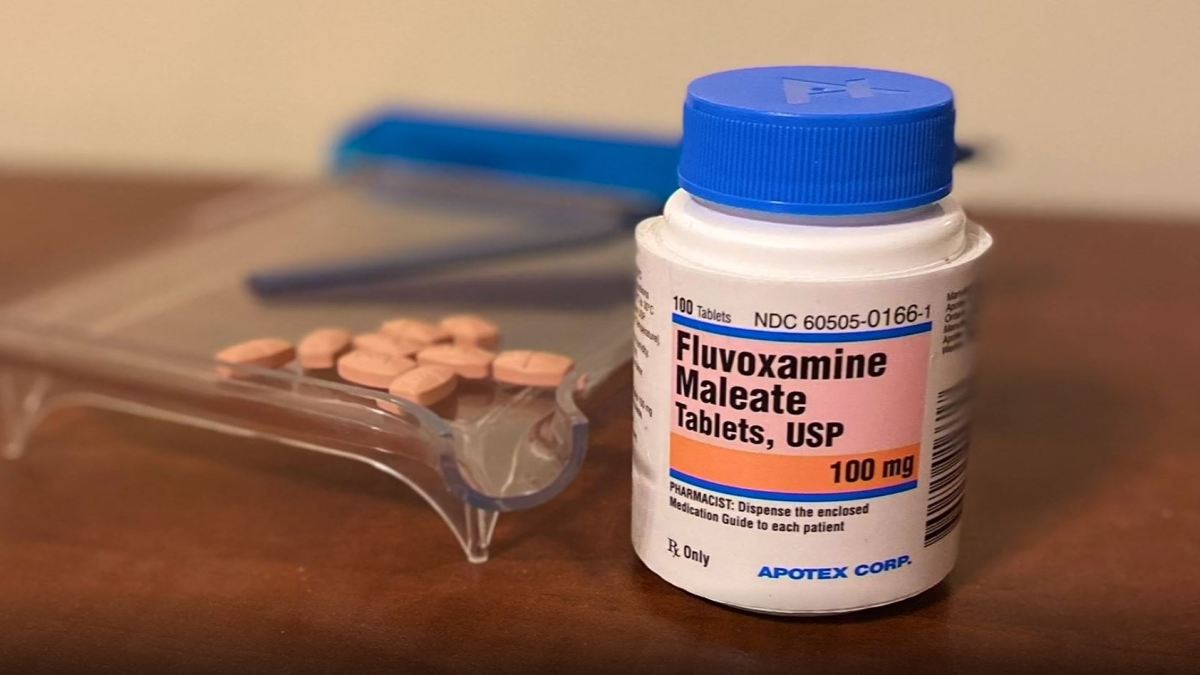
A commonly prescribed pill – approved more than 13 years ago by the Food and Drug Administration for depression and obsessive-compulsive disorder – shows initial success in preventing people with COVID-19 from developing severe symptoms and be hospitalized.
The drug Fluvoxamine, sold under the brand name Luvox, appears to prevent inflammation in the lungs of people infected with COVID-19, which can be fatal.
“What we observed was that all of the patients who received fluvoxamine, none of them had a serious COVID infection that was affecting their lungs or respiratory status,” said Dr. Caline Mattar , an infectious disease researcher at Washington University in St. Louis who helped conduct an initial trial of the drug last fall.
Now the University of Washington, along with Northwestern University, University of Minnesota, University of Washington, and University of Utah, is conducting a larger trial of Fluvoxamine, giving a two-week course on the medication to patients.
“I feel much better: I didn’t have a fever; I didn’t have chills; the congestion is gone,” said Eduardo Veliz of Los Angeles, who tested positive for COVID-19 earlier this month, and agreed to be part of the larger trial on fluvoxamine.
Veliz told the I-Team that he started to feel a lot better after five days of trying. “My taste came back,” Veliz said.
Fluvoxamine – which costs around $ 10 for a two-week supply – has recently shown real results in preventing serious COVID illness.
Last November, at Golden Gate Fields racetrack in Berkeley, 200 workers tested positive for COVID-19. The runway doctor, Dr David Seftel, had learned of the initial success of fluvoxamine and offered it to the infected workers.
On Monday, Dr Seftel published the promising results of his treatment in the journal Oxford Academic. Sixty-five workers took fluvoxamine for two weeks, and none developed severe symptoms of COVID-19, and none required hospitalization.
But of the 48 employees who refused the drug, 60% developed symptoms and 12.5% had to be hospitalized.
Some seniors have received their first dose of the COVID-19 vaccine, but many are concerned about receiving a second dose on time. Joel Grover reported on NBC4 News on Tuesday, January 26, 2021.
Researchers say there are great benefits to testing existing drugs approved by the FDA on COVID patients, also known as drug “reuse.”
“They’ve been around for a while, we know they’re safe, they’re available, they tend to be relatively cheaper,” said Dr. Mattar of the University of Washington.
Critical care doctors who treat patients with COVID are excited about the prospect of having more drugs that could keep people from overcrowded hospitals.
“I hope I’m optimistic about fluvoxamine… now this needs to be carried over into larger trials in real patients,” said Dr. Raj Dasgupta of USC’s Keck Hospital.
Real patients, like Eduardo Veliz of Los Angeles, are also optimistic, although he is unsure whether he is taking fluvoxamine or the placebo given to some trial participants.
“Whatever I take, it helps me,” Veliz told the I-Team.
“Our goal is to help patients who are initially well enough to be at home and prevent them from getting sick enough to be hospitalized,” Dr Mattar told NBC4.
COVID patients who wish to participate in the larger Fluvoxamine trial can find out more at StopCovidTrial.wustl.edu
[ad_2]
Source link