
[ad_1]
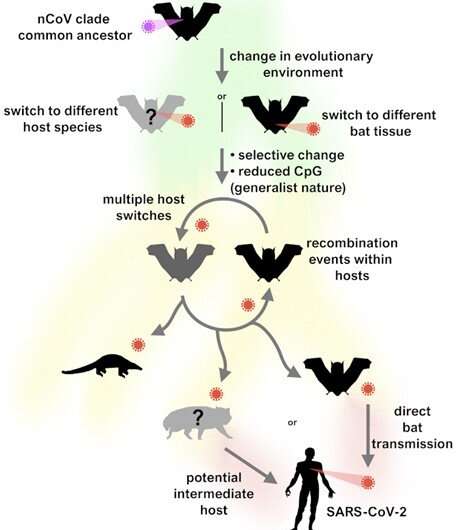
Diagram of our proposed evolutionary history of the nCoV clade and putative events leading to the emergence of SARS-CoV-2. Credit: MacLean OA, et al. (2021), Natural selection in the evolution of SARS-CoV-2 in bats has created a generalist virus and a highly capable human pathogen. PLoS Biol 19 (3): e3001115. CC-BY
How much has SARS-CoV-2 had to change to adapt to its new human host? In a research article published in the open access journal PLOS biology Oscar MacLean, Spyros Lytras from the University of Glasgow and their colleagues show that since December 2019 and during the first 11 months of the SARS-CoV-2 pandemic, there have been very few ‘significant’ genetic changes observed in the hundreds of thousands of sequenced virus genomes.
The study is a collaboration between researchers from the United Kingdom, the United States and Belgium. Lead authors, Professor David L. Robertson (at MRC-University of Glasgow Center for Virus Research, Scotland) and Professor Sergei Pond (at Institute for Genomics and Evolutionary Medicine, Temple University, Philadelphia) were able to draw on their experience in data analysis. from HIV and other viruses to SARS-CoV-2. Pond’s advanced analytical framework, HyPhy, was instrumental in uncovering evolutionary signatures embedded in virus genomes and is based on decades of theoretical knowledge of molecular evolutionary processes.
The first author, Dr Oscar MacLean, explains: “This does not mean that no changes have occurred, evolutionarily unimportant mutations accumulate and ‘ride’ along millions of transmission events, as they do. do it in all viruses. ” Some changes can have an effect; for example, the replacement of Spike D614G which has been shown to improve transmissibility and some other adjustments in viral biology scattered throughout its genome. Overall, however, “neutral” evolutionary processes dominated. MacLean adds: “This stasis can be attributed to the highly sensitive nature of the human population to this new pathogen, with limited pressure from population immunity and a lack of containment, leading to exponential growth making almost all virus a winner. “
Pond comments: “What has been so surprising is how transmissible SARS-CoV-2 has been from the start. Usually viruses that jump to a new host species take a while to adapt to. be as capable of spreading as SARS-CoV-2, and most never get past this stage, resulting in dead-end spillovers or localized epidemics.
By studying the mutation processes of SARS-CoV-2 and related sarbecoviruses (the SARS-CoV-2 group of viruses belong to bats and pangolins), the authors find evidence of a fairly large change, but all before the emergence of SARS-CoV-2 in humans. This means that the “ generalist ” nature of many coronaviruses, and their apparent ease of jumping between hosts, imbued SARS-CoV-2 with a ready-made ability to infect humans and other mammals, but these properties have probably evolved in bats before the overflow. to humans.
Co-author and Ph.D. student Spyros Lytras adds: “Interestingly, one of the closest bat viruses, RmYN02, has an intriguing genomic structure made up of both SARS-CoV-like segments -2 and bat virus. Its genetic material carries both distinct compositional signatures (associated with the action of the host’s antiviral immunity), this evolutionary rhythm change has occurred in bats without the need for an intermediate animal species. “
Robertson comments, “The reason for the ‘shift in speed’ of SARS-CoV-2 in terms of its increased rate of evolution at the end of 2020, associated with more highly mutated lines, is that the immunologic profile of the human population has modified.” The virus towards the end of 2020 came increasingly into contact with the host’s existing immunity, with the number of people previously infected now high. This will select variants that can dodge part of the host’s response. Associated with the escape of immunity in long-term infections in chronic cases (eg, in immunocompromised patients), these new selective pressures increase the number of important viral mutants.
It is important to understand that SARS-CoV-2 remains an acute virus, cleared by the immune response in the vast majority of infections. However, it is now moving faster away from the January 2020 variant used in all current vaccines to increase protective immunity. Current vaccines will continue to work against most variants in circulation, but the more time passes and the greater the gap between the number of people vaccinated and unvaccinated, the more opportunities there will be to escape the vaccine. Robertson adds: “The first race was to develop a vaccine. The race now is to get the world’s population vaccinated as quickly as possible.”
Understanding the evolution of SARS and COVID-19 viruses
Oscar A. MacLean et al, Natural selection in the evolution of SARS-CoV-2 in bats has created a generalist virus and a highly capable human pathogen, PLOS biology (2021). DOI: 10.1371 / journal.pbio.3001115
Provided by Public Library of Science
Quote: SARS-CoV-2 passed from bats to humans without much change (2021, March 12) retrieved March 13, 2021 from https://phys.org/news/2021-03-sars-cov- humans.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link