[ad_1]
Moderna announced Monday that it has started administering its next-generation COVID-19 vaccine candidate in a Phase 1 study. The new candidate, mRNA-1283, can be stored in a refrigerator, which could potentially reduce obstacles to storage and shipping.
“We are pleased to begin this Phase 1 study of our next-generation COVID-19 vaccine candidate, mRNA-1283,” Stéphane Bancel, CEO of Moderna, said in a press release on Monday. “Our investments in our mRNA platform have enabled us to develop this next generation vaccine candidate which is a potential refrigerator stable vaccine that could facilitate distribution and administration in a wider range of settings, potentially including for developing countries. We remain committed to helping respond to this ongoing public health emergency. “
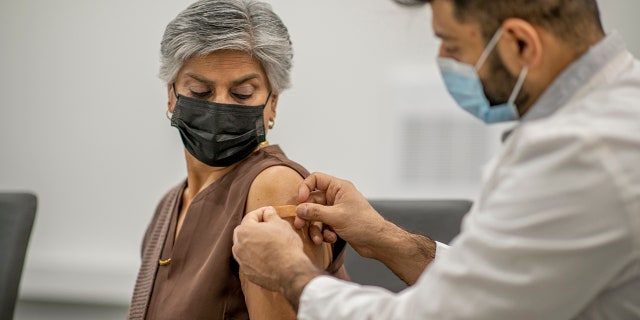
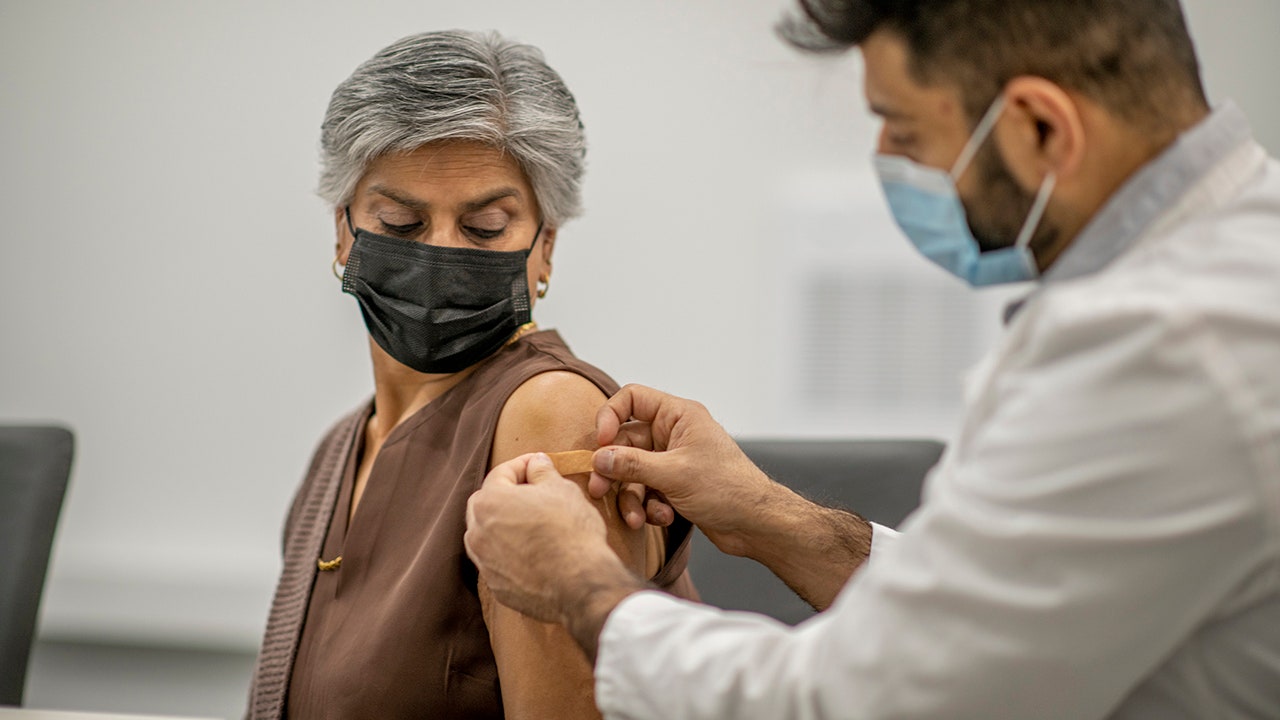
The new clinical trial evaluates three dose levels of mRNA-1283, which targets the peak SARS-CoV-2 protein, given to healthy adults in a series of two doses spaced 28 days apart and at a level dose in one shot.
(iStock)
The currently approved Moderna two-dose vaccine can be stored in the refrigerator between 2 degrees Celsius and 8 degrees Celsius for up to 30 days before use, but cannot be refrozen once thawed. Unperforated vials can be stored between 8 degrees Celsius and 25 degrees Celsius for up to 12 hours, but they also cannot be refrozen.
PFIZER COVID-19 ‘PARTIAL’ VACCINATION REDUCES THE RISK OF INFECTION BY 63% IN NURSING RESIDENTS: CDC
The new clinical trial evaluates three dose levels of mRNA-1283, which targets the peak SARS-CoV-2 protein, given to healthy adults in a series of two doses spaced 28 days apart and at a level dose in one shot. The results will be compared to the dose level currently permitted in the two-dose series.
CLICK HERE FOR FULL CORONAVIRUS COVERAGE
“MRNA-1283 is intended to be evaluated in future studies for use as a booster dose for previously vaccinated or seropositive individuals as well as in a primary series for seronegative individuals,” said the press release.
[ad_2]
Source link