[ad_1]
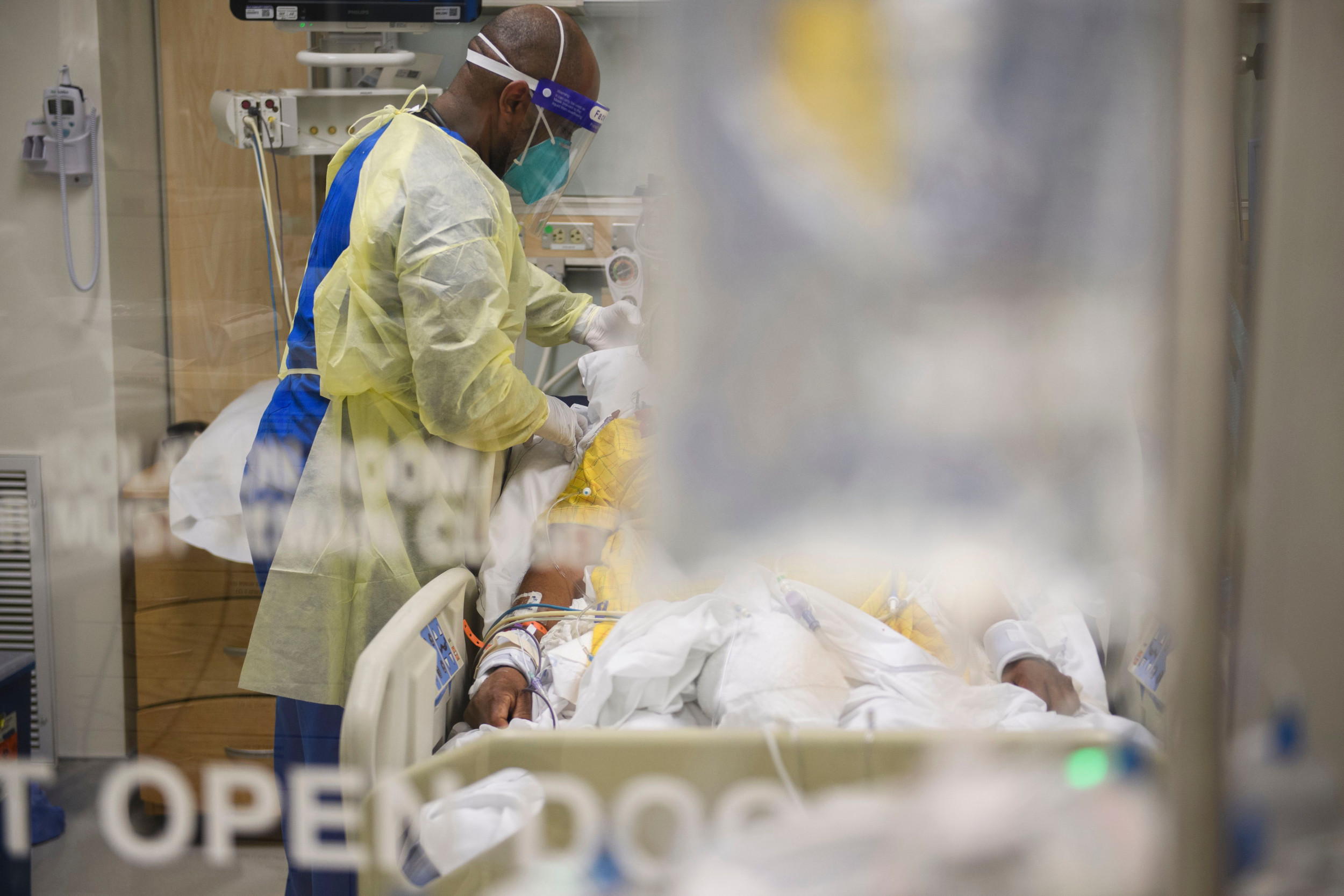
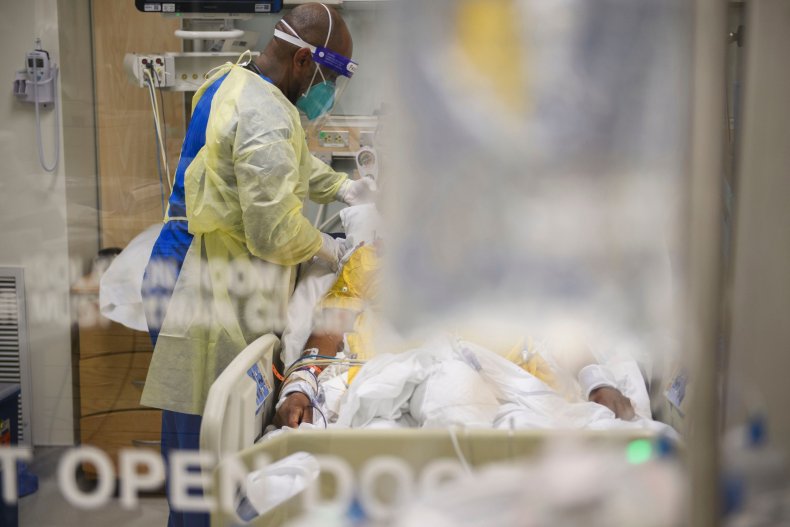
As his job approval rating plummets and the Delta variant of Covid19 still rages on, President Biden tonight presented new guidelines – including vaccine mandates for companies with 100 or more employees – for try to control the virus.
For a group of companies and medical researchers scattered around the world, the speech was yet another disappointment after a busy year. For them, Biden’s speech represented a missed opportunity to promote a simple, inexpensive and widely available tool to fight the virus: nasal sprays.
How can nasal sprays help fight COVID 19? The virus and its variants infect patients primarily by adhering to the nasal membrane as the original source of infection, says Nathan Jones, CEO of Xlear, the nasal spray maker based in American Fork, Utah, as well as ‘a range of dental care. some products.
PATRICK T. FALLON / AFP via Getty Images
Researchers who have studied the issue say that any spray that works by blocking the adhesion of the virus to the nasal membrane and physically washing the virus out of the nose, will likely be just as effective with the Delta variant. Sanotize, a biotechnology company based in Vancouver, Canada, has started phase three trials in several countries of its spray to assess its effectiveness against Delta. Preliminary trials have been promising, according to the company, and the sale has already been provisionally approved by public health agencies in Israel and New Zealand. The company hopes the phase three trials will be completed by the end of the year.
As Biden’s speech on Thursday night illustrates, the US approach to COVID has centered on vaccines. This has been extremely frustrating for a company like Xlear, which has entered into a year-long dialogue with the Centers for Disease Control and the Food and Drug Administration, seeking emergency use authorization for its nasal spray as a COVID treatment. . (In order to make antiviral claims in its advertising and marketing, a company needs an EUA from the FDA.) It also tried to get the Centers for Disease Control to issue guidelines on the use of nasal sprays. to mitigate the impact of the coronavirus.
So far, the FDA and CDC have resisted these pleas. In a long letter to the company sent this summer and seen by News weekSandra Cashman, executive secretary of the CDC chief of staff’s office, calls one of the independent studies on the effectiveness of the nasal spray “small”, says she discusses the effectiveness of the nasal spray only in terms of treatment of symptoms, and “shows no hard evidence … in terms of reducing viral load.”
The company has submitted several studies which it says show that its spray helps “destroy the virus,” as Jones puts it. Other researchers point to a study published in the New England Journal of Medicine that shows the viral load is concentrated in the nose and upper respiratory tract, something, Jones says, “we know since February 2020, and we still don’t talk not about that. “
But for the CDC, it was not enough. “If other data is published [on viral load reduction] The CDC will review this additional scientific evidence to… determine updates to our recommendations, ”Cashman wrote. the agency believes it hasn’t seen enough reliable data, so it won’t update its guidelines. But when she does, she will. “
Despite the failure of the CDC, XLear’s dialogue with the FDA continued throughout the summer, prompting some optimism that an EUA could be possible at some point. A recent letter outlined some steps the company needed to take to move towards an EUA and stressed that it was always open to dialogue. “So that was at least a positive point,” said an attorney for Xlear working on the matter.
But that’s about all that was positive. The FDA made requests that seemed strange to some people associated with Xlear. For example, the spray produced by Xlear is made from grapefruit seed extract. The FDA wanted to know the origin of the grapefruits the company uses, according to the sources, to the fields in which they were grown and the day they were harvested. But the company buys from a supplier, who in turn buys the grapefruits from another company. Establishing where and when specific grapefruits were harvested is going to be a nightmare.
The FDA has also requested a safety trial. But over-the-counter nasal sprays have been on the market for years with almost no serious safety concerns. “It just doesn’t make sense,” said a source familiar with the company’s communications with the agency. “They want mountains from us and a molehill from the drug makers.”
Jones expressed his frustration. “We are not a fringe group of people who are just looking for simple solutions through a nasal spray. We know we are not a quick fix. But we can be a weapon in this fight, “he says – and a profitable weapon:” nasal spray solutions cost $ 6 a month or even less. “

Jessica McGowan / Getty Images
Six months ago, hoping to understand the agency’s internal deliberations on nasal sprays and their potential to fight COVID, Xlear filed an Freedom of Information request with the CDC. He got the results earlier this month. The CDC turned 558 pages, three of which were redacted. “There is literally nothing there,” said a lawyer familiar with the case. “We had asked them to look at the use of nasal sprays, but there was no research, no study discussed. No one was even assigned to anyone.”
The CDC has said it is and will remain responsive, and is awaiting the FDA’s response on the EUA before issuing guidance.
[ad_2]
Source link