[ad_1]
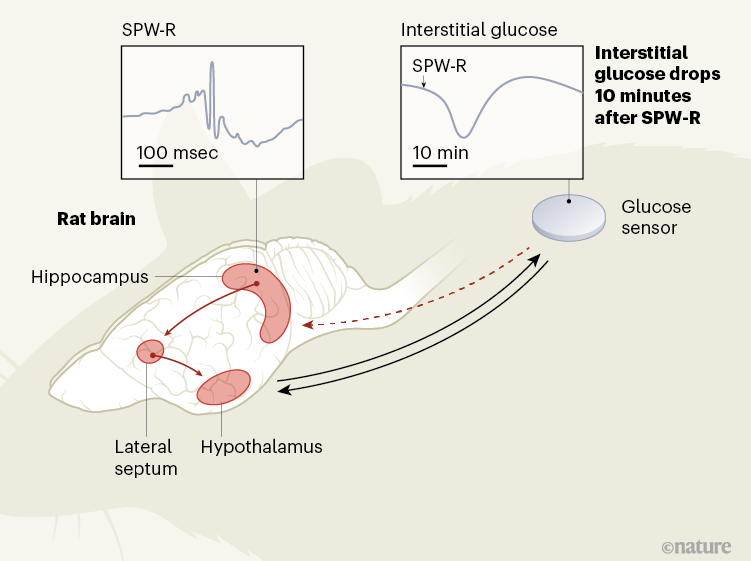
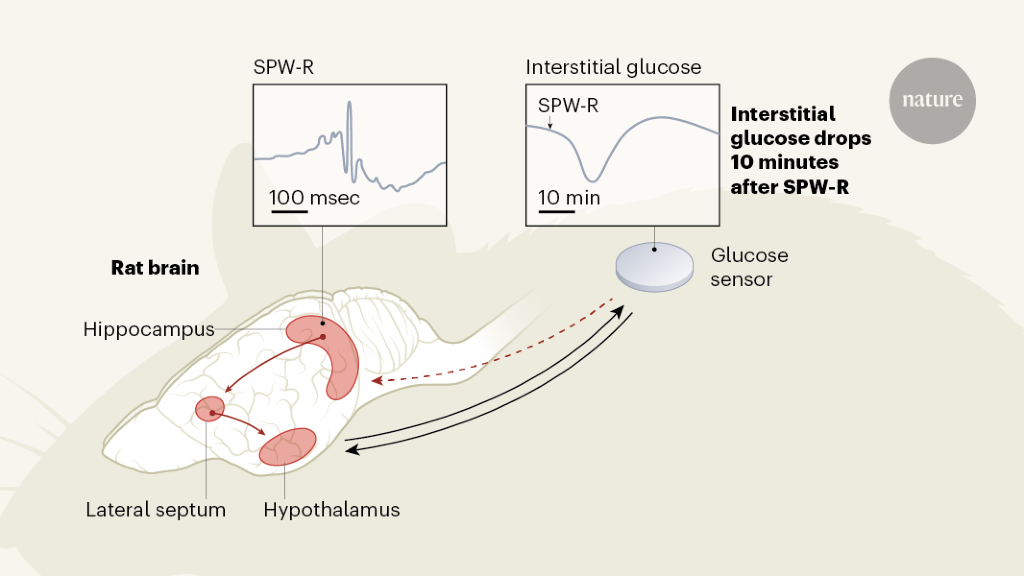
To regulate adaptive behavior, the brain relies on a continuous flow of cognitive and memory-related processes that require a constant energy supply. Weighing about 1,200 grams in women and 1,300 grams in men, the brain consumes on average about 90 grams, or 340 kilocalories, of glucose per day, which is about half of the body’s demand for glucose.1,2. The tight integration of metabolic and cognitive signals could help match the brain’s energy supply to its energy needs, optimizing foraging behavior and efforts to limit energy expenditure. The synchronization of glucose supply with brain activity has heretofore been thought to be a function of a structure called the hypothalamus, at the base of the brain. Write in Nature, Tingley et al.3 provide evidence in rats of the role of another region of the brain, called the hippocampus, which is usually involved in memory and navigation, in this equation (Fig. 1).
The hippocampus receives many types of sensory and metabolic information, and the projections of neuronal cells from the hippocampus extend to various parts of the brain, including the hypothalamus. Thus, the hippocampus could indeed represent a hub in which metabolic signals are integrated into cognitive processes.3. To examine this possibility, Tingley and his colleagues recorded oscillatory patterns called sharp wave ripples (SPW-Rs), reflecting changes in electrical potential across cell membranes of neuronal cell sets in the hippocampi of rats. They did this by using a sensor inserted under the skin of the animals’ backs to continuously measure glucose levels in the interstitial fluid surrounding the cells.
SPW-Rs are composed of a sharp wave, originating from neurons in the CA3 region of the hippocampus, which causes a rapid but localized network oscillation – the ripple – in the CA1 and connected regions. Basically, SPW-Rs are associated with synchronous bursts of neuronal discharges and represent a hallmark of cognitive processing of experience, particularly related to memory.4.
The authors demonstrated that pooled SPW-Rs recorded in a part of the hippocampus called dorsal CA1 are followed by a marked drop in peripheral glucose concentrations approximately 10 minutes later. Although the level of SPW-R, which averaged nearly 10 per minute, varied widely over a 24-hour cycle, the authors show that a reduction in glucose concentrations of about 0.33 milligrams per deciliter has emerged by SPW-R. SPW-Rs that were large in amplitude but short in duration and that were clustered over time predicted the most pronounced drops in glucose.
This striking observation suggests a previously unknown role of SPW-Rs as a phase reset signal to control an animal’s glucose levels. Some aspects of this proposed role require consideration, including the timing of observed glycemic responses. The delay of about 10 minutes between SPW-Rs and the drop in glucose could be explained by factors such as the slow rate of diffusion of glucose into the interstitial space and a technical delay inherent in the glucose reading of an implanted sensor. Therefore, the coupling of the hippocampus and peripheral glucose levels via SPW-Rs could be even closer.
Another aspect to consider is the direction of the change in glucose levels after SPW-Rs. These oscillatory patterns are associated with increased shooting activity in the hippocampus, as well as in various parts of the cerebral cortex that receive the hippocampal outputs. Therefore, given this increased discharge and, consequently, possibly increased brain energy demands, one would expect the hippocampal signal to cause an increase, rather than a decrease, in intake. glucose. In stressful situations, the brain can quickly signal – via neurons in the sympathetic nervous system – to the rest of the body to increase peripheral glucose levels. However, during SPW-R, the brain regions that are involved in sympathetic activation appear to be deactivated.5. Could it then be that the glucose reductions after SPW-Rs simply reflect the increased uptake of the brain and the “pull” of peripheral glucose to the brain during SPW-Rs?
Smart control experiments by Tingley and colleagues suggest this is not the case. They found that artificial inhibition of the lateral septum, an important relay station for the signal from the hippocampus to the hypothalamus, abolished the coupling of SPW-Rs with peripheral glucose fluctuations. This finding, and the observation that artificially induced hippocampal ripples were also followed by drops in glucose, supports the idea that a signal specifically associated with SPW-R, and which is transmitted through the hypothalamus, induces drops in blood glucose. availability of glucose in the periphery of the body. .
These findings could generate new ideas on how the regulation of glucose supply to the brain is integrated with the homeostatic regulation of glucose turnover in the whole animal. Additionally, since the SPW-R-glucose coupling was the same when animals were fasting as when they could eat freely, these results shed new light on the hypothesis that brain systems control blood glucose levels in the body. normal conditions.6, and not just in the face of stress and other metabolic challenges. Future studies should determine whether the drops in glucose associated with SPW-R occur throughout the animal or reflect a redistribution of energy that prioritizes the brain.7, and should identify the underlying mechanisms.
SPW-Rs are not mere companions to large increases in neuronal discharge activity: during these patterns, the hippocampus re-enacts, in a time-ordered fashion, experiences that were previously encoded in memory. SPW-Rs do not occur when a rat is actively exploring a maze. Instead, they occur during short rests between periods of locomotion, when the rat seems to “plan” where to go next. They also occur during episodes of slow sleep (sleep characterized by low-frequency electrical oscillations) after exploratory behavior, when lasting memories of the experience need to be fixed. When replaying the SPW-R, traces of pre-existing information can be combined to influence decisions4. Thus, it appears that the SPW-Rs characterize an “offline” mode in which the hippocampus and connected areas of the brain engage in “thought-like” activity.
With this in mind, Tingley’s discoveries et al. to link discrete periods of thought-like reprocessing of previous experiences to immediate metabolic regulation. Since SPW-Rs also coordinate hippocampal activity with the activity of sets of neurons in cortical exit areas of the hippocampus, they could even allow large parts of the brain to exert cognitive control over the hippocampus. peripheral metabolism in offline mode. Recent findings suggest that thought-type memory-related activity, as reflected by SPW-Rs and which typically appears in times of no acute stress, may benefit from small, synchronized gaps in energy supply.8. Indeed, this could be a first clue as to why hippocampal SPW-Rs should downregulate, rather than upregulate, peripheral glucose concentrations.
In summary, the intriguing results of Tingley and colleagues point to the existence of a hitherto unknown hippocampal loop that underlies cognitive control of blood sugar regulation. The authors’ findings not only open new perspectives for basic research, but also invite a reconsideration of various clinical phenomena. These include the co-occurrence of cognitive decline and, in particular, deterioration of the hippocampus with impaired glucose regulation in type 2 diabetes.9; the association of slow sleep disorders with insulin resistanceten; and the disruption of food intake in people with hippocampal lesions and associated memory disorders11. It is tempting to speculate that the loss of metabolic control under these conditions could have a common cause in the dysfunctional expression of hippocampal SPW-Rs.
Competing interests
The authors declare no competing interests.
[ad_2]
Source link