
[ad_1]
Haotian Wang, an engineer at Rice University, adjusts the electrocatalyst reactor built in his lab to recycle carbon dioxide to produce liquid fuel. The reactor is designed to be an efficient and cost effective way to reuse the greenhouse gas and keep it out of the atmosphere. Credit: Jeff Fitlow / Rice University
The "green" invention of Rice University reduces carbon dioxide into a valuable fuel.
A common greenhouse gas could be reused in an efficient and environmentally friendly way with an electrolyser that uses renewable electricity to produce pure liquid fuels.
The catalytic reactor developed by Haotian Wang, an engineer in chemical engineering and biomolecular at Rice University, uses carbon dioxide as a raw material and, in his latest prototype, produces highly purified and high concentrations of formic. acid.
The formic acid produced by traditional carbon dioxide devices requires expensive and energy-intensive purification steps, Wang added. Direct production of pure formic acid solutions will help promote commercial carbon dioxide conversion technologies.
The method is detailed in Nature Energy.
Wang, who joined Rice's Brown School of Engineering in January, and her group are studying technologies to turn greenhouse gases into useful products. During testing, the new electrocatalyst achieved an energy conversion efficiency of about 42%. This means that almost half of the electrical energy can be stored in formic acid in the form of liquid fuel.
"Formic acid is a vector of energy," Wang said. "It's a fuel cell fuel that can generate electricity and emit carbon dioxide – which you can recover and recycle.
"It's also fundamental in the chemical industry as a raw material for other chemicals and as a hydrogen storage material that can hold nearly 1,000 times the energy of an equivalent volume of hydrogen, which is difficult to compress, "he said. "This is currently a big challenge for hydrogen fuel cell cars."
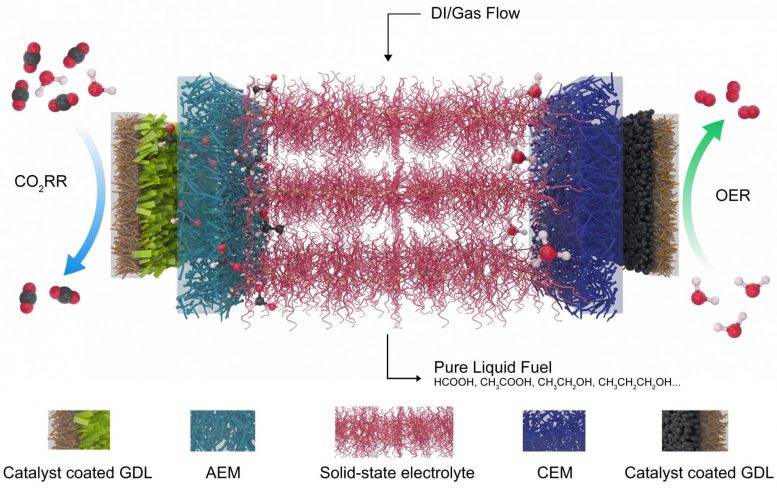
This diagram shows the electrolyser developed at Rice University to reduce carbon dioxide, a greenhouse gas, into valuable fuels. On the left, a catalyst that selects carbon dioxide and reduces it to a negatively charged formate, which is driven through a gas diffusion layer (GDL) and the anion exchange membrane (AEM) into the atmosphere. central electrolyte. On the right, an Oxygen Release Reaction Catalyst (OER) generates positive protons from the water and sends them through the cation exchange membrane (CEM). The ions recombine into formic acid or other products that are extracted from the system by deionized water (DI) and gas. Illustration of Chuan Xia and Demin University Liu / Rice
Two advances made the new device possible, said senior author and postdoctoral researcher Rice, Chuan Xia. The first was to develop a robust two-dimensional bismuth catalyst and the second to use a solid-state electrolyte that eliminates the need for salt as part of the reaction.
"Bismuth is a very heavy atom, compared to transition metals like copper, iron or cobalt, "said Wang. "Its mobility is much lower, especially in reaction conditions. This stabilizes the catalyst. He noted that the reactor was structured to prevent water from coming into contact with the catalyst, which also contributes to its preservation.
Xia can manufacture nanomaterials in bulk. "Currently, people produce catalysts on a milligram or gram scale," he said. "We have developed a way to produce them per kilogram. This will facilitate the intensification of our process for the industry. "
The polymer-based solid electrolyte is coated with sulfonic acid ligands to conduct positive charges or amino-functional groups to drive negative ions. "Usually, people reduce the carbon dioxide contained in a traditional liquid electrolyte, such as salt water," Wang said. "You want the electricity to be driven, but the electrolyte of pure water is too resistant. You must add salts such as sodium chloride or potassium bicarbonate so that the ions can move freely in the water.
"But when you generate formic acid that way, it mixes with salts," he said. "In most applications, you have to remove the salts from the final product, which requires a lot of energy and cost. We have therefore used proton-conducting solid electrolytes which may consist of insoluble polymers or inorganic compounds, thus eliminating the need for salts. "
The rate at which water passes through the product chamber determines the concentration of the solution. Slow flow with the current configuration produces a solution containing nearly 30% by weight of formic acid, while faster flow rates allow customization of the concentration. Researchers expect to reach higher concentrations for next-generation reactors that accept gas flow to produce pure formic acid vapors.
Rice has collaborated with the Brookhaven National Laboratory to visualize the process underway. "X-ray absorption spectroscopy, a powerful technique available on the Inner Shell Spectroscopy light line (ISS) of the Brookhaven Lab National Light Synchrotron Light Source II, allows us to probe the electronic structure of electrocatalysts in operation. , that is to say during the actual chemical process. "Said co-author Eli Stavitsky, scientist in charge of light lines at ISS. "In this work, we followed the oxidation states of bismuth at different potentials and were able to identify the active state of the catalyst during the reduction of carbon dioxide."

An electrocatalyst reactor built at Rice University recycles carbon dioxide to produce liquid fuel solutions based on electricity. The scientists behind the invention hope that it will become an efficient and cost effective way to reuse the greenhouse gas and keep it out of the atmosphere. Credit: Jeff Fitlow / Rice University
With its current reactor, the laboratory generated formic acid continuously for 100 hours with negligible degradation of reactor components, including nanoscale catalysts. Wang suggested that the reactor could be easily re-tooled to produce higher value products such as acetic acid, ethanol or propanol.
"The overall picture is that reducing carbon dioxide is very important for its effect on global warming as well as for the synthesis of green chemicals," Wang said. "If electricity comes from renewable sources like the sun or the wind, we can create a loop that turns carbon dioxide into an important substance without emitting more."
###
The co-authors are Peng Zhu, graduate rice student; graduate student Qiu Jiang and Husam Alshareef, Professor of Materials Science and Engineering at the University of Science and Technology King Abdullah, Saudi Arabia (KAUST); Postdoctoral researcher Ying Pan of Harvard University; and scientist Wentao Liang from Northeastern University. Wang is an Assistant Professor in Chemical and Biomolecular Engineering with William Marsh Rice Trustee. Xia is a J. Evans Attwell-Welch Postdoctoral Fellow at Rice.
Rice and the Office of Science User Facilities of the United States Department of Energy supported the research.
Reference: "Continuous production of pure liquid fuel solutions by electrocatalytic CO2 reduction using solid electrolyte devices", by Chuan Xia, Peng Zhu, Qiu Jiang, Ying Pan, Wentao Liang, Eli Stavitsk, Husam N Alshareef and Haotian Wang, September 2, 2019, Nature Energy. DOI: 10.1038 / s41560-019-0451-x
Abstract
Electrocatalytic CO2 the reduction is often carried out in an electrolyte solution such as KHCO3(aq), which allows ionic conduction between the electrodes. As a result, the liquid products that form are in mixture with the dissolved salts, requiring energy intensive downstream separation. We report here the continuous electrocatalytic conversion of CO2 solutions of pure liquid fuel in cells using solid electrolytes, where electrochemically generated cations (such as H+) and anions (such as HCOO–) are combined to form solutions of pure products without mixing with other ions. Using a Bi selective catalyst for HCOOH (faradic efficiency> 90%) and easily dimmable at the cathode, we demonstrate the production of pure HCOOH solutions with a concentration up to 12 M. We also show a continuous generation and stable for 100 h of 0.1M HCOOH with degradation of selectivity and activity. Production of other C without electrolyte2+ liquid oxygen solutions, including acetic acid, ethanol and notpropanol, are also demonstrated using a Cu catalyst. Finally, we show that our CO2 The solid electrolyte reduction cell can be modified to accommodate other more complex practical applications.
[ad_2]
Source link