[ad_1]
New research suggests that there could be another force, even stronger than a hydrogen bond, keeping our DNA together.
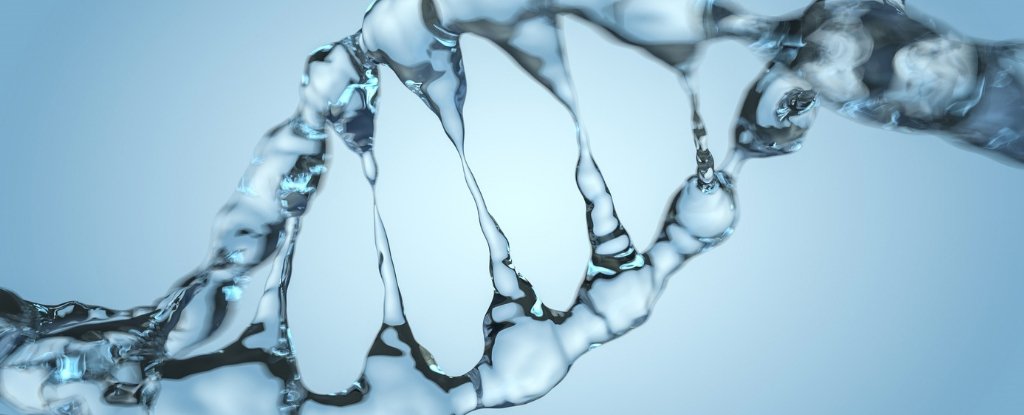
Discovered for the first time in the 1950s, the double helix structure of our genetic material has become iconic, although we still do not know how all its pieces come together.
Similar to a twisted scale, the bars are pairs of nitrogen bases, held together by some of the most powerful intermolecular attractions: hydrogen bonds.
Linking the two sides of the scale, these ultra-strong links are often described as the main stabilizing force of DNA. But perhaps there is a bigger factor at stake.
DNA replication is usually done with the help of several enzymes that "unpack" the DNA molecules by breaking their hydrogen bonds. It turns out, however, that this is not the only way to destabilize the double helix.
By testing DNA in a more hydrophobic environment than normal, researchers at the Chalmers University of Technology in Sweden have now shown for the first time that this water-repellent force alone was enough to solve the double helix.
"The main stabilizer of the DNA double helix is not the hydrogen bonds base pairs, but the stacking of base pairs in heap," the authors conclude, "whose hydrophobic cohesion , requiring abundant water, would indirectly dry out the interior of the DNA so that the hydrogen bonds can exercise its full power of recognition ".
In other words, as the DNA base pairs are naturally water-repellent, in a normal water solution, they pile up to stay safe, much like a penguin colony packed.
It's hard to know if enzymes in nature are doing something similar, but given other similar models, the team thinks it's a possibility separate.
The splitting of these groups requires the opposite effect. By gradually adding a hydrophobic solution of polyethylene glycol – which is often used in cars as antifreeze – the team has shown that DNA loses its structure and that this occurs as soon as its environment goes from water to water repellent treatment.
"The cells want to protect their DNA and not expose it to hydrophobic environments, which can sometimes contain harmful molecules," says chemical engineer and lead author Bobo Feng.
"But at the same time, the cell's DNA must open to be able to be used."
As such, Feng and her colleagues propose that a normal cell keeps its DNA in an aqueous solution until it wants to read, copy or repair its DNA. It is only then that the cell creates a more hydrophobic environment through the use of enzymes with a function similar to that of polyethylene glycol.
 (Yen Strandqvist / Chalmers Technology University)
(Yen Strandqvist / Chalmers Technology University)
Steven Brenner, molecular biophysicist at NASA, told ScienceAlert that it was an important discovery demonstrating a new way for enzymes to "melt" the DNA double helices. for transcription or repair. Nevertheless, he warns, the way in which many media have covered this document is not entirely accurate.
Despite what many say, the results do not suggest that hydrogen bonds are unimportant for DNA formation. Only these hydrophobic forces also play a crucial role.
And it's not a new concept. Models that include hydrophobic interactions in the double helix date back at least to the 1990s, and nowadays whole labs are devoted to this line of research.
In 1997, scientists began to question the notion that hydrogen bonds alone can maintain both strands of a DNA double helix together. This explanation, it seems, was insufficient and several years later, in 2004, a study revealed that a hydrogen bond was not necessary for the stability of the base pairs.
Just a few years ago, in 2017, a study showed that a lack of complementary hydrogen bonds does not really bother cells and that synthetic bases are nevertheless transcribed and successfully translated, using only hydrophobic forces. .
Together, these results suggest that the forces we observed in nature are not the only ones responsible for the double helix.
"It would be very easy to say that complementary hydrogen bonds are what defines DNA and RNA," said biochemist Floyd Romesberg, author of the 2017 paper.
"But we have found that forces other than hydrogen bonding can participate productively in every step of information storage and retrieval."
Yet, although we have learned over the years, the conclusions we can draw from these models still have limitations.
"One of the sad lessons of the physical organic chemistry of the last century," Benner told ScienceAlert, "is that the efforts made to separately model the behavior of molecules as a consequence of different factors … says longer on the chemist than on modeling tells you about the molecules themselves. "
These frameworks, for example, can be evaluated either on their ability to simply Explain DNA or on their ability to actually make he. Personally, Benner believes that the last analysis is more objective, because explanations are often enough to convince us that we understand what is happening.
"However, if our models actually allow us to do things, then they really have to have a reality behind them," he argues.
In the end, Benner explains that hydrogen bonding and hydrophobicity have been found to be necessary for making natural DNA, and that this two-part model is currently used in both human medicine and life research. alien by NASA.
The new research adds to this idea by providing a possible biological mechanism for this process.
"Nobody had previously placed DNA in a hydrophobic environment like this one and studied its behavior, so it 's no wonder no one has discovered it until the end of the day. now, "said Feng.
The results were published in PNAS.
[ad_2]
Source link