
[ad_1]
Matter takes many forms, but most of us know the three basic states: solid, liquid and gaseous. For the first time, scientists have discovered that it is possible that the material exists simultaneously in two of these states.
In particular, the metallic potassium can be a solid and a liquid simultaneously, if you treat it exactly. Just apply extreme pressure and extreme temperature and presto! You have a piece of potassium that is both solid and melted.
"It would be as if you were holding a sponge filled with water that is starting to sink, except that the sponge is also made of water," said physicist Andreas Hermann of the University of Toronto. Edinburgh to Adam Mann. National Geographic.
Potassium is quite simple. It has a basic crystalline crystalline structure in its solid form. But subject to extreme conditions, strange things can happen to simple metals.
We know, for example, that sodium, a conductive metal, becomes a high-pressure insulator. Lithium becomes a superconductor at high pressure and low temperature.
 (McBride et al., Phys Rev. B, 2015)
(McBride et al., Phys Rev. B, 2015)
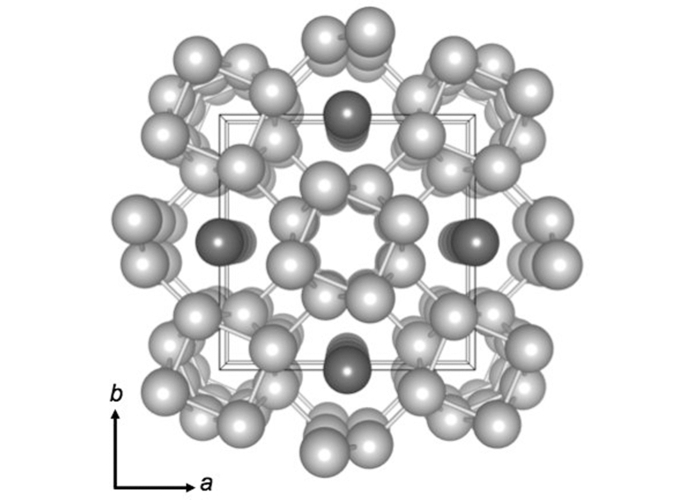
Previous experiments with high pressure potassium showed that its atoms organized into a complex arrangement – five tubes of atoms in a square formation, four at the corners and one in the middle; and four chains of atoms linked together.
When heat is applied, the chains disappear; the researchers termed this the "chain fusion transition", supposed to occur when potassium chains pass from an ordered state to a disordered state.
In an attempt to understand why this was happening, researchers used powerful computer simulations in the latest study to observe the behavior of about 20,000 atoms of potassium under extreme conditions.
When the pressure and temperature are sufficiently high (about 2 to 4 gigapascals), the potassium atoms are organized into chains and lattices connected together.
The chemical interactions between the atoms of the lattice are strong, so that they remain solid ordered when a temperature between 400 and 800 Kelvin is applied. But during this time, the chains melt into a messy and liquid state.
The team describes this new state as a "chain melting phase" and thinks it can exist in various materials, including sodium and bismuth, under the right conditions, which are probably different from the conditions required to induce this state. potassium.
"Potassium is one of the simplest metals we know, but if you squeeze it, it forms very complicated structures," Hermann said.
"We have shown that this unusual but stable state is both solid and liquid.Recreation of this unusual state in other materials could have all kinds of applications."
The research must be published in PNAS.
[ad_2]
Source link