[ad_1]
Cancer can come back when a subset of tumor cells, called persistent cells, survive chemotherapy. Most of these lingers do not divide (quiescent) in the presence of the therapeutic drug, but a rare subpopulation may re-enter the cell cycle during treatment, allowing them to proliferate. Much research has focused on the genetic mechanisms underlying such resistance to treatment. However, emerging data suggests that non-genetic mechanisms (such as changes in the complex of DNA and protein called chromatin) may also play a role in the development of a persistent condition. Write in Nature, Oren et al.1 examine cell lines and gene expression profiles of persistent cells using a method called DNA bar coding to trace tumor cells and their descendants. Their findings shed light on the role of reversible non-genetic mechanisms in chemotherapy resistance for a range of tumors from different tissues.
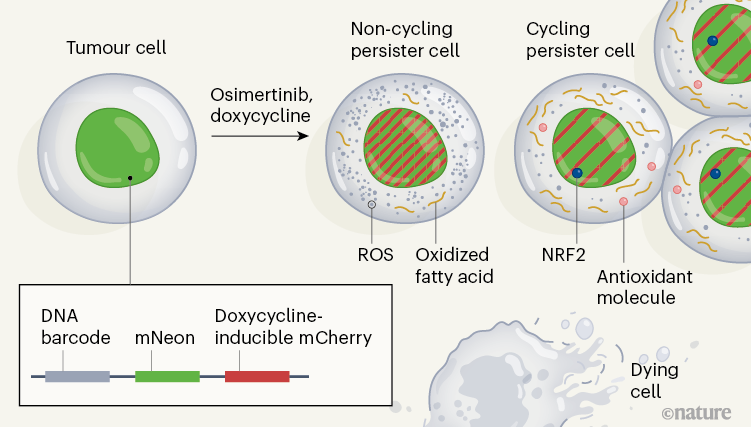
The authors analyzed cell divisions in cultured human lung cancer cells in vitro which have a mutation in the gene encoding the epidermal growth factor receptor (EGFR). The cells were treated with osimertinib, an inhibitor of this receptor. Oren and his colleagues followed the results of cell lines from the tumor cell line and found that 8% of the lines gave rise to persistent cells after 14 days, and 13% of the persistent ones resumed the cell cycle and proliferated to form cell colonies. . These results show that these persistent cyclable and non-cyclic occur early in treatment, and that they evolve from distinct cell lines.
To characterize the molecular mechanisms associated with persistent cycling and non-cyclic cells, the authors developed a system they call Watermelon, to simultaneously trace the lineage, proliferation state and transcriptional state of each cell (Fig. 1). . To determine whether the persistent condition was due to an irreversible genetic property of the persistent cells, the authors re-exposed the population of persistent cells to osimertinib after a treatment break. They found that cells in both cyclic and non-cyclic populations reacquire drug sensitivity, suggesting that a non-genetic reversible mechanism underlies persistence.
The authors assessed gene expression using the single-cell RNA sequencing method at different times during a two-week treatment, and compared these signatures in persistent cyclic and non-cyclic. The persistent state of the cycle was uniquely characterized by the upregulation of defense programs that produce antioxidant molecules – including expression signatures characteristic of the metabolism of the antioxidant glutathione, as well as the production of the NRF2 protein, which is a transcription factor induced in response to oxidative stress. Additionally, expression of multiple NRF2 target genes correlated with lines that had a large number of persistent descendant cells, and genetic engineering of the cells to deplete a negative regulator of NRF2 resulted in an increase in the fraction of persistent cells that were cycling.
Treatment with osimertinib induced the formation of reactive oxygen species (ROS), which can cause oxidative stress. At the end of treatment, persistent cyclists had significantly lower ROS levels than persistent non-cyclists. When the authors decreased the levels of ROS in cells through the addition of ROS trapping molecules, the fraction of persistent cells that cycled increased. These analyzes therefore suggest that the redox state of cells has a role in the regulation of persistent cycling.
Recognizing that redox balance is related to metabolism, the authors profiled the products of metabolism in persistent cyclics and non-cyclics, and identified 56 products that differed in abundance between these two cell populations. The authors found a greater abundance of fatty acids bound to the carnitine molecule (result of a step prior to the oxidation of fatty acids) in cycling states than in non-cycling states. The authors also noted an increase in fatty acid oxidation following treatment with osimertinib. Modulation of the pathway affecting fatty acid oxidation revealed that increasing or decreasing fatty acid oxidation results in an increase or decrease in the cycle persistency fraction, respectively. These results support the idea that a metabolic change in fatty acid oxidation affects the proliferative capacity of the persistent.
To test whether their observations extended beyond the model lung cancer system, Oren et al. generated watermelon models of other types of human cancer, using melanoma, lung, breast and colorectal tumors. They treated the cells with appropriate inhibitors, characteristic of chemotherapy drugs, based on the genetics underlying the particular cancer. In most of these models, the persistent cyclics showed high fatty acid metabolism, antioxidant responses, and NRF2 signatures compared to the non-cyclic persistents, showing that the authors’ findings extend to cancer types other than cancer. lung cancer.
Those in vitro the results were validated using a modified mouse model in which the animals had an inducible version of a mutant EGFR in lung tumors. After osimertinib treatment, persistent cells exhibited a higher level of ROS and gene expression signatures characteristic of fatty acid metabolism compared to cells from untreated mice. The authors also assessed changes in gene expression before and after chemotherapy in cell samples from people with EGFR-induced lung adenocarcinoma, with melanoma induced by a mutant version of the BRAF enzyme (treated with BRAF and MEK enzyme inhibitors), and with breast cancer induced by a mutant version of the HER2 protein (treated with lapatinib). In all of these scenarios, the signatures of ROS production and fatty acid metabolism were increased in persistent cells after treatment compared to untreated tumor cell samples, and were higher in cycles than in persistent non-cycles. .
The study of Oren and his colleagues is part of the larger context of current work highlighting the importance of non-genetic mechanisms in the survival and proliferation of persistent cells.2–4. A major problem when studying the persistent is that they represent only a small fraction of the initial population of tumor cells, which makes it difficult to characterize them by sequencing the cells in bulk. The interest of the authors’ Watermelon method is that it allows the detailed characterization of the persists at the resolution of single cells. A future direction could be to apply similar single-cell approaches to study non-genetic mechanisms of resistance in other types of cancer, such as pancreatic cancer.5 or prostate6 tumors, which are areas where such research is emerging.
Understanding the dynamics of persistent cells is crucial for the development of more effective chemotherapies for cancer treatment. Previous studies have shown that the estrogen hormone response pathway7, which is involved in breast cancer, and the pathway in the cell death process called ferroptosis8,9 are associated with the persistent state. Oren et al. found that although inhibition of these pathways reduced the amount of persistent cells, there was an increase in the fraction of persistent cells that cycled, suggesting that these would not be optimal chemotherapy targets.
In contrast, the authors report that inhibition of the fatty acid oxidation pathway using the inhibitor drug etomoxir resulted in a decrease in both the fraction of persistent cells and the fraction of persistent cells that cycled. This promising result indicates that the modulation of this pathway, and genes that have functions related to this pathway, could be of interest to consider in the development of new treatment strategies.
Competing interests
The authors declare no competing interests.
[ad_2]
Source link