[ad_1]
The immune signaling associated with inflammation is sometimes considered something to be avoided. However, immune signaling can also be protective, suppressing damage and pathogenic microorganisms (pathogens). In the brain, several types of cells work together to maintain brain health, mediate inflammatory responses, and optimize the function of major output cells, neurons. Write in Nature, McAlpine et al.1 discover a signaling axis between two of these types of brain cells, astrocytes and microglia. They demonstrate that this signaling, mediated by the immune protein interleukin-3 (IL-3), limits disease progression and brain dysfunction in a model of Alzheimer’s disease.
Alzheimer’s disease (AD) is a devastating and widespread neurodegenerative disease that results in the loss of brain cells and synaptic connections between neural cells, leading to progressive cognitive decline. One of the hallmarks of AD is the presence of disease-associated aggregates of different proteins in the brain: that is, plaques made up of amyloid-β (Aβ) protein and neurofibrillary tangles made up of tau protein.2.
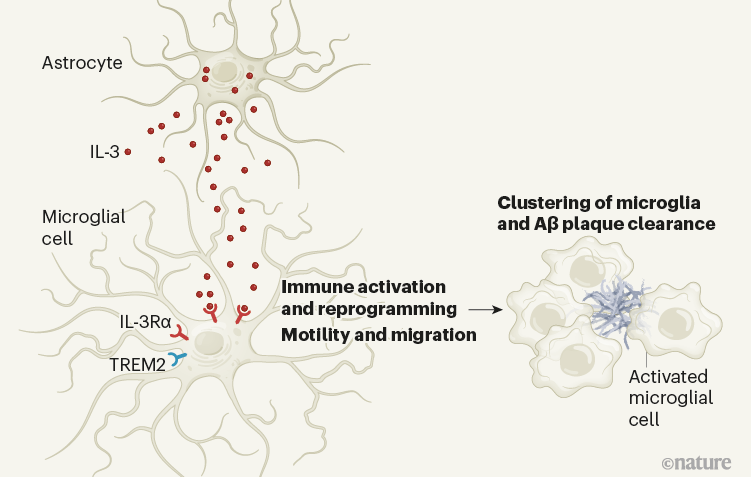
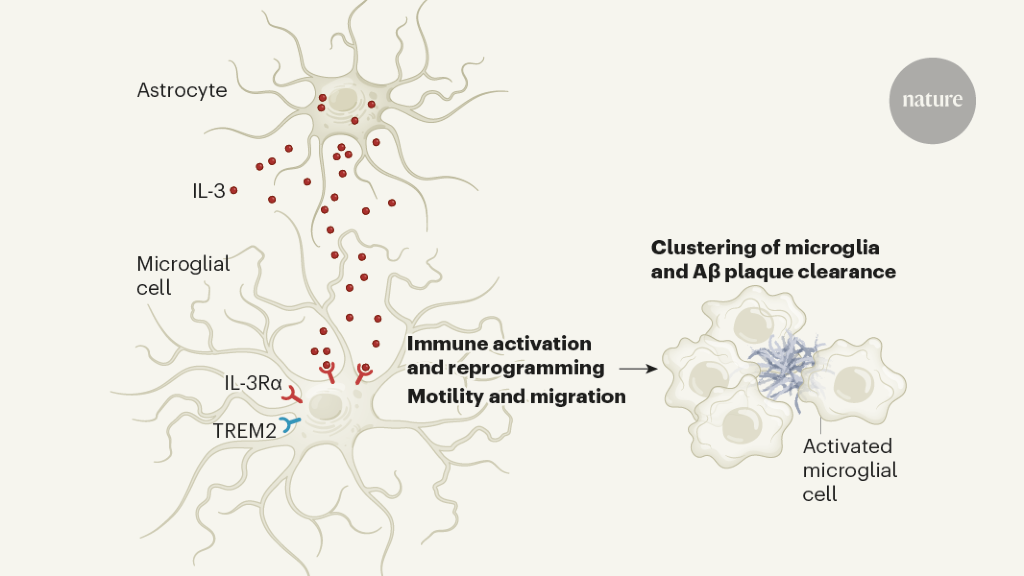
Normally, astrocytes and microglia help maintain neuronal health and function by removing debris, recycling neurotransmitter molecules, and promoting communication between synapses.3. In the brain of a person with AD, astrocytes and microglia activate, produce inflammatory molecules, and cluster around protein plaques. This microglial aggregation can be protective, by preventing loose (soluble) Aβ protein from diffusing throughout the brain4. However, the signals that coordinate the functions of the microglia are not fully understood. McAlpine et al. show that the cytokine IL-3 produced by astrocytes is one of these signals, and that it plays a central role in a model of AD.
Cytokines are soluble signaling proteins that are a key form of immune communication. They are involved in complex signaling loops that can initiate, intensify or resolve inflammation, recruit immune cells where they are needed, and aid in the removal of pathogens and cellular debris. Their impact on the brain has long been of interest to neurological diseases in which inflammation is observed along with alterations in neural function.
The roles of IL-3 in the brain have not been particularly well studied. However, it is known to regulate inflammation in several ways, for example by causing the proliferation of immune cells, including those that circulate in the blood.5, and it has been associated with the risk of AD in plasma studies of patients6,7.
McAlpine et al. examined a model of AD in which mice carry five mutations that have been implicated in the disease in humans. These AD mice develop Aβ plaques and exhibit progressive impairment of short-term memory with age8. When these AD model mice also lacked IL-3, they exhibited increased accumulation of Aβ plaques, higher levels of soluble Aβ, and greater impairments in short-term and spatial memory.
To identify cellular sources of IL-3 in the brain, McAlpine et al. mice generated in which IL-3 producing cells are fluorescently labeled; this approach identified a subset of astrocytes as a major source of IL-3. AD mice in which IL-3 was specifically deleted from astrocytes showed increased accumulation of Aβ plaques and more severely impaired short-term memory compared to AD mice. The authors observed a similar effect in AD mice that lacked IL-3 completely, suggesting that astrocytes are the key cellular reservoir for the protein in this AD model (Fig. 1). In particular, the aggregation of microglia near the Aβ plaques was reduced in AD mice lacking astrocytic IL-3.
The authors then identified the targets for IL-3 in the brain. They found that microglia expresses the IL-3 IL-3Rα receptor, and that levels of this receptor increase dramatically with age and in the AD model compared to young wild-type mice. Suppression of IL-3Rα specifically from microglia in AD mice resulted in effects on A and plaque load and memory similar to those seen in AD mice lacking IL-3 in astrocytes.
Strikingly, McAlpine and colleagues found that injecting IL-3 into the brains of AD mice could reduce Aβ buildup and stimulate microglia clustering around Aβ plaques (Fig. 1). Continuous administration of IL-3 into the brains of IL-3 deficient AD mice for four weeks resulted in a remarkable reduction in the size and quantity of plaques and soluble Aβ levels, as well as improvements in short-term memory compared to results observed in AD mice injected with an inactive control substance. This is a key discovery with potential therapeutic implications.
Interest in the role of microglia in AD has increased dramatically since the discovery that a variant of the gene encoding the TREM2 receptor protein, which is expressed by microglia, is associated with the risk of AD9. McAlpine et al. CA watch Il3ra is enriched in a previously described subset of “disease associated microglia” which are activated by the TREM2 receptorten. In addition, the authors found that the removal of Trem2 prevented the increase in microglial expression of IL-3Rα in their AD model, raising the question of whether TREM2 mutations associated with AD risk in humans could prevent this IL-3 dependent protective response.
Indeed, the authors also found evidence that this pathway is at work in the human brain. In the brain tissue of individuals who died of AD, the authors observed higher astrocyte expression of IL-3 and microglial expression of IL-3Rα than in the brain of individuals of the same age without AD. In addition, the amount of microglial expression of IL-3Rα was correlated with the length of time these individuals had been diagnosed with AD, as well as the accumulation of Aβ plaques.
How does IL-3 promote the protective functions of microglia? The authors found that in AD mice lacking IL-3, microglia did not cluster around the plaques and that plaque deposition was greater than in AD mice. In experiments with cultured human microglia, treatment of these cells with IL-3 promoted migration to aggregates of AD-associated proteins.
It is important to keep in mind that IL-3 can offer protection through several mechanisms. For example, IL-3 was particularly abundant in astrocytes at the blood-brain barrier, which controls the passage of proteins and cells from the circulatory system to the brain. Populations of immune cells called macrophages, which reside at the borders of the brain, may have been targeted by some of the genetic tools used to manipulate IL-3Rα expression. Such cells could also be affected by IL-3 to modify the entry of molecules or cells into the brain. Although McAlpine et al. examined some aspect of the integrity of the blood-brain barrier and found it intact, other unmeasured variables such as the active transport of blood-borne molecules to the brain may be affected11.
In addition, it is possible that other types of brain cells express the IL-3 receptor in certain contexts and respond to IL-3. Further dissecting the impact of IL-3 signaling on other cellular players will be a critical next step.
Nonetheless, these findings represent an exciting advance in understanding the role of astrocytes and microglia in AD, a disease notoriously difficult to treat and currently lacking in curative or restorative therapies. Although caution should be exercised in translating these findings in the clinic, especially given the role of IL-3 and IL-3Rα in certain autoimmune diseases12, this study raises the intriguing idea that IL-3 or related molecules may have therapeutic potential in AD. Could this be a step towards personalized therapy for people with AD who wear a TREM2 variant? Further defining the roles of IL-3 in health and disease will be essential to deliver on the promise of McAlpine and his colleagues’ findings.
Competing interests
The authors declare no competing interests.
[ad_2]
Source link