
[ad_1]
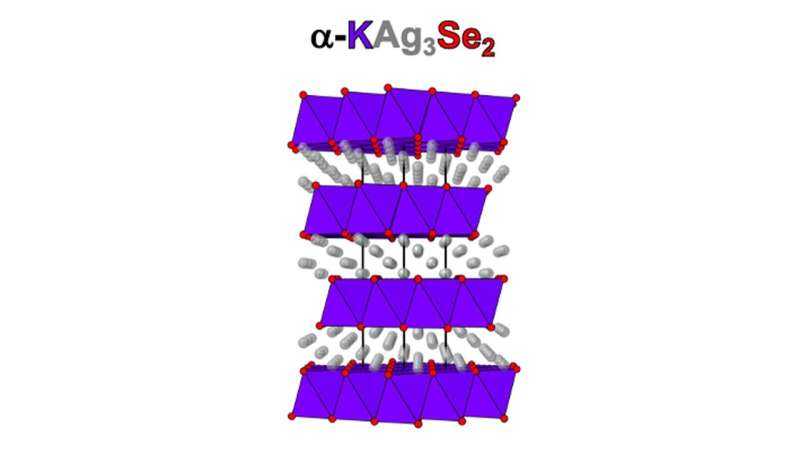
Four-layer atomic structure of -KAg3I know2, a 2D superionic conductor. The colors of the atoms are coordinated with the colors of the name. Credit: Mercouri Kanatzidis / Northwestern University and Argonne National Laboratory
An unforeseen discovery could lead to crucial future discoveries in batteries, fuel cells, devices for converting heat to electricity and more.
Scientists normally conduct their research by carefully selecting a research problem, devising an appropriate plan to solve it, and executing that plan. But unexpected discoveries can happen along the way.
Mercouri Kanatzidis, a professor at Northwestern University with a joint appointment at the US Department of Energy’s (DOE) Argonne National Laboratory, was looking for a new superconductor with unconventional behavior when he made an unexpected discovery. It was a material that is only four atoms thick and allows the study of the motion of charged particles in just two dimensions. Such studies could stimulate the invention of new materials for a variety of energy conversion devices.
“Our analysis results revealed that before this transition, the silver ions were fixed in the confined space in the two dimensions of our material, but after this transition they stirred,” explains Mercouri Kanatzidis, co -assignment with Argonne and Northwestern University.
The target material for Kanatzidis was a combination of silver, potassium, and selenium (α-KAg3I know2) in a four-layer structure like a wedding cake. These 2D materials have length and width, but almost no thickness at just four atoms high.
Superconducting materials lose all resistance to the movement of electrons when cooled to very low temperatures. “To my disappointment, this material was not a superconductor at all, and we weren’t able to make one,” said Kanatzidis, senior researcher at Argonne’s Materials Science Division (MSD). “But to my surprise, this turned out to be a fantastic example of a superionic conductor.”
In superionic conductors, charged ions of a solid material flow as freely as in liquid electrolytes in batteries. This results in a solid with unusually high ionic conductivity, a measure of the ability to conduct electricity. This high ionic conductivity is accompanied by low thermal conductivity, which means that heat does not pass easily. These two properties make superionic conductors super materials for energy storage and conversion devices.
The team’s first clue that they had discovered a material with special properties was when they heated it to between 450 and 600 degrees Fahrenheit. It moved to a more symmetrical layered structure. The team also found that this transition was reversible when it lowered the temperature and then raised it again in the high temperature zone.
“Our analysis results revealed that prior to this transition, the silver ions were fixed in the confined space in the two dimensions of our material,” Kanatzidis said. “But after that transition, they got restless.” While much is known about how ions move in three dimensions, very little is known about how they do so in only two dimensions.
Scientists have been looking for an exemplary material for studying the movement of ions in 2D materials for some time. This potassium-silver-selenium layered material appears to be one. The team measured how ions diffused through this solid and found it to be equivalent to that of a strongly salted water electrolyte, one of the fastest known ionic conductors.
While it is too early to say whether this particular superionic material could find any practical application, it could immediately serve as a crucial platform for the design of other 2D materials with high ionic conductivity and low thermal conductivity.
“These properties are very important to those designing new two-dimensional solid electrolytes for batteries and fuel cells,” said Duck Young Chung, principal materials scientist at MSD.
Studies with this superionic material could also be instrumental in the design of new thermoelectrics that convert heat into electricity in power plants, industrial processes and even the exhaust fumes of automobile emissions. And such studies could be used to design membranes for environmental cleaning and water desalination.
This research appeared in a Natural materials.
New material offers environmentally friendly solution to convert waste heat into energy
Alexander JE Rettie et al, A type I two-dimensional superionic conductor, Natural materials (2021). DOI: 10.1038 / s41563-021-01053-9
Provided by the Argonne National Laboratory
Quote: A super material applicable to batteries and other energy conversion devices (2021, September 14) retrieved on September 15, 2021 from https://phys.org/news/2021-09-super-material-applicable-batteries-energy .html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link