[ad_1]

The precise imaging of individual molecules has been brought to a new level, with a demonstration that electrons can be delivered to them one by one and the resulting changes in the molecular structure discerned in the images.1
This technique has been described by Leo Gross and his colleagues at IBM's research labs in Zurich, Switzerland. The results are "impressive and convincing," says Daniel Ebeling of Justus Liebig University in Giessen, Germany, who did not participate in the research. He adds that this technique "can provide fundamental information that goes far beyond a mere improvement in image resolution and should allow us to increase our understanding of surface reaction processes".
Gross's team has already shown that the detection of molecules adsorbed on surfaces using an atomic force microscope (AFM), to which is fixed a molecule of carbon monoxide (CO), can provide images with incredibly high resolution. They previously used this method to image unique molecules that are subject to chemical reactions and charge transfer processes.
To visualize charged states, the researchers placed the molecules on an insulating surface, for which they used very thin films of sodium chloride deposited on copper. They cooled the system to 5K to suppress the motions and vibrations of the molecules and used a CO-end AFM tip to image various organic molecules under ultrahigh vacuum.
Gross and his colleagues could transfer the charge from one electron to another to individual molecules by applying voltage to the tip. They measured how the force on the tip varied with tension – a method called Kelvin probe force microscopy. Step jumps in force when the voltage has been changed indicate a change in state of charge.
The Gross team has loaded azobenzene, an organic molecule commonly used as a photomechanical molecular switch, because it can be transformed between cis and trans isomers. They found that in the neutral trans state that the two benzene rings at each end of the molecule are inclined out of the plane in the same direction, but the addition of one electron causes the inclination of one cycle in the other direction, resulting in a twist . Calculations of molecular structure using functional density theory (DFT) predict the same behavior.
The researchers also loaded pentacene molecules: linear chains of five fused benzene rings. They could solve slight changes in the length of bonds on different rings because a two-electron charge changes the bond pattern, the different carbon-carbon bonds changing their bonding orders – usually between single and double – in different locations.

In the case of the tetracyanoquinodimethane molecule (TCNQ), meanwhile – a powerful electron acceptor often used in molecular electronics – the charge of the neutral molecule has switched from a standing conformation to the surface at a flat position, while modifying the molecule binding. This change in orientation as a function of state of charge has not been seen before, and according to Mr. Gross, this effect "could be useful as a molecular switch in a device based on the jumps of an electron to the other".
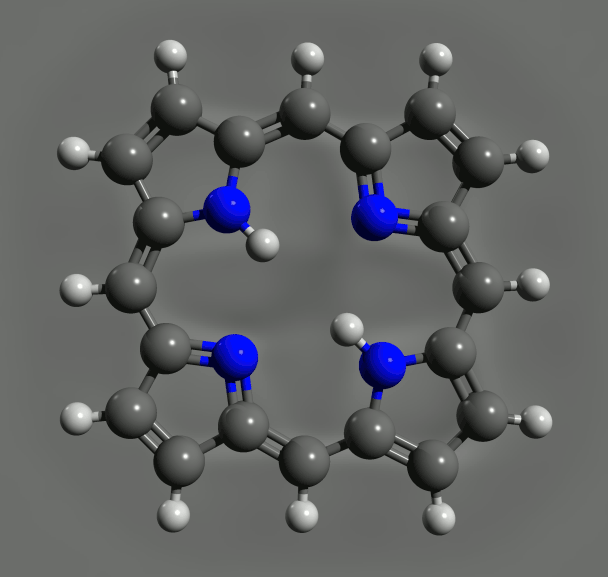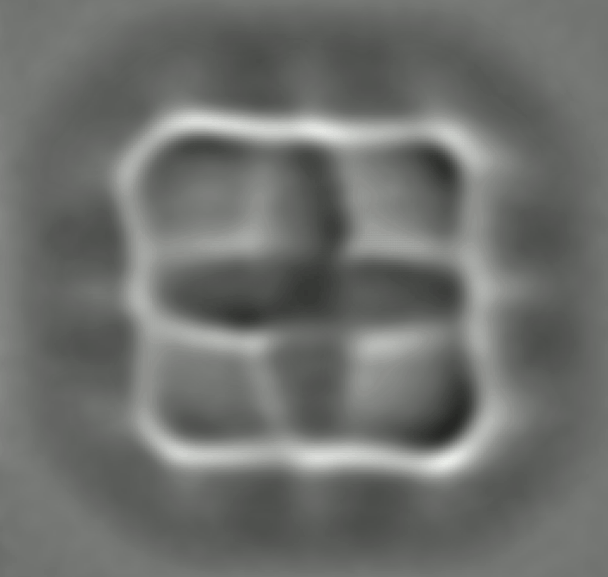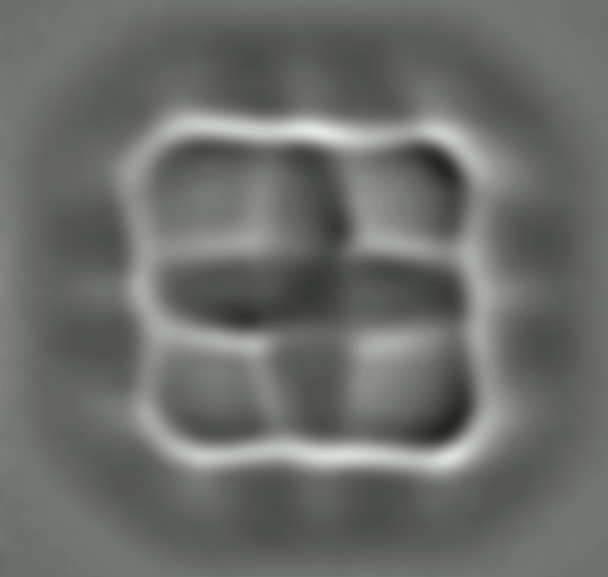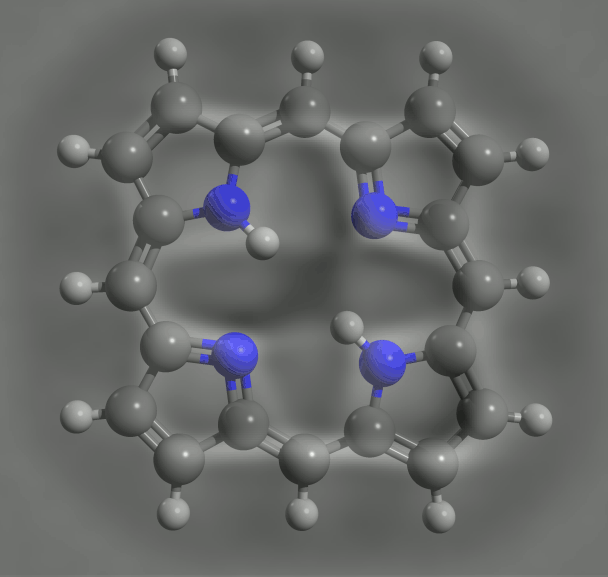
By loading a ring of porphyrin – the key molecular group of chlorophyll, where light absorption leads to charge transfer – the IBM team found link changes due to the effects of conjugation, consistent with again with DFT calculations. Such an agreement is reassuring, but does not make the experiments redundant. DFT relies on various assumptions and approximations. So, although "often, it's okay, we can not recover," says Gross. Nikolaj Moll, a member of the IBM team, specializing in such calculations, says that "comparing experiences with the results of the DFT can help to find the limits of the theory and to improve it."
The ability to analyze the order of bonds in these experiments on charged molecules is impressive, says Yoshiaki Sugimoto of the University of Tokyo in Japan, a specialist in molecular imaging. "The combination of imaging with state of charge control and sub-molecular resolution imaging opens the way for accurate characterization of charged molecules," he said.
Gross says the technique could also help synthesize new molecules and molecular devices through the manipulation of atoms. His team has already used the AFM method to make a molecule difficult to synthesize by traditional means. It has recently shown that by creating states of multiply charged molecules, they can form and break bonds.2 "Identifying radical sites like pentacene and resolving structural changes during loading will help us better understand and predict these new synthetic routes," he said.
[ad_2]
Source link