[ad_1]
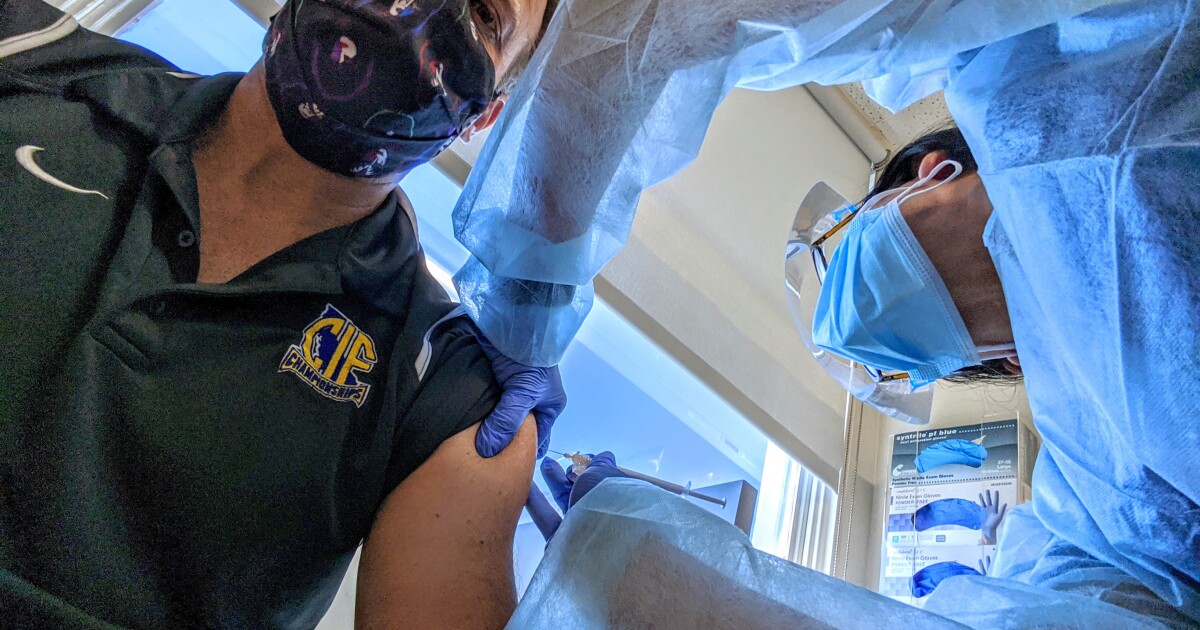
Three months ago, Dan Stepenosky, a colon cancer survivor, volunteered to help test a vaccine in development to stop the spread of the coronavirus. Last month, his teenage daughter Kearston joined him in the lawsuit.
For the 52-year-old educator, a few weeks of aches and pains was a small price to pay to be part of the vanguard leading the way in the fight against COVID-19.
Today, Calabasas’ father and daughter relish the news that the vaccine they received – developed by Pfizer and tested locally by Kaiser – was 90% effective in protecting people from transmission of the virus in trials global. It may be in production at the end of the year.

Dan Stepenosky said he agreed to participate in the vaccine trial despite being a cancer survivor because “I believe in science”.
(Mel Melcon / Los Angeles Times)
“I guess we made the right bet,” Stepenosky said, as the news broke. “I hated staying here the whole month of April, May and June, watching the news and feeling like I could do something.”
He heard about a doctor friend’s trial last summer.
“I applied, but I thought they would reject me, that the cancer would kill me,” he said. But he passed the application process and got the first of his two-dose regimen on August 31. It was a double-blind study, so he has no idea if his vaccine was real or a placebo.
The process itself is not particularly difficult.
“They give you a chance, watch you for half an hour,” then send you home with an app to check you in each week and report any symptoms, Stepenosky said. “If you develop any symptoms, they have a self-swab kit that they send directly to UPS to pick up.”
There are six office visits for blood tests to check if the participant is making antigens. He still has three visits to make.
Beyond that, participants are encouraged to live their lives normally, he said.
“They don’t want someone who is going to stay home and be isolated,” he said. “It won’t be good if you don’t go out, if you quarantine yourself.”
::
Stepenosky was a little reluctant to tell his friends and colleagues that he had agreed to test the vaccine.
“I didn’t want to scare anyone,” he says. He had a bit of hindsight, even from his brother, a radiologist. “He said, ‘Why are you doing this? What’s your offer? ‘”
Some people thought he shouldn’t take unnecessary risks.
“Some people say, ‘This is so awesome, I’m amazed.’ And some people say, “You are an idiot”. But Stepenosky is a former high school physics teacher who is not swayed by the opinions of others about the coronavirus. “I believe in science and I believe in medicine, so I totally agree.”
He was actually hoping for side effects when the vaccine went into effect.
“I didn’t want the placebo,” he says.
But when his muscles started to ache a few days later, the pain was so mild: “I didn’t know if it was just because I was 52, or if I was thinking too much about it,” he says. he. The pains went up and down, “and for three consecutive weeks I wondered, this is [the vaccine] do that, or is my body just playing tricks?
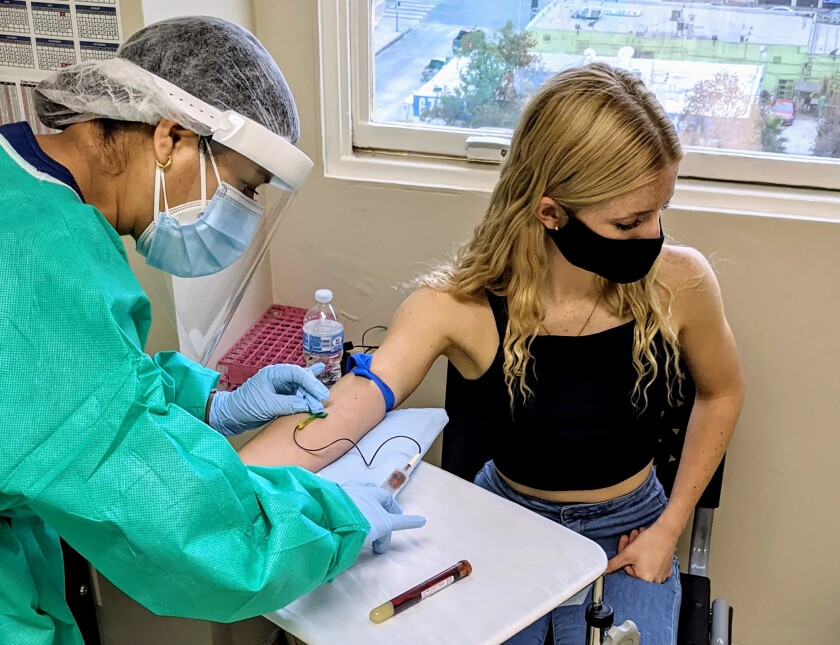
Kearston Stepenosky said of his vaccine testing experience: “Being able to play the guinea pig for such an important science has been very motivating for me.
(Dan Stepenosky)
He resisted so calmly that his daughter, Kearston, 16, also wanted to take the leap.
She’s a Calabasas High junior, honorary student and captain of the basketball team, who is determined to “do all I can to be that tiny little stat that helps us get back to school and to go back to basketball. ”
Her father was not on board initially. “He was afraid,” she said, “that I was missing too much school.
But her mother, Sharon, spoke with medics and encouraged Kearston to follow through.
“I have some faith in organizations – in Kaiser and Pfizer,” said Sharon Stepenosky. “Someone has to do it, if we are to move the process forward. I think it’s great, it’s brave. So when the vaccine becomes available, people may not be afraid of it.
For his daughter, the fallout from the vaccine was rather humiliating. “I kind of approached the subject with the mentality that it could be fun,” Kearston admitted sadly. “I was clearly a little naive.”
She found herself ill and exhausted for days, with headaches, chills and body aches. “I was not afraid,” she insisted. “It was embarrassing, more than anything.”
Still, for a young woman aiming for a medical career, the experience was well worth it.
“It was great to see the trial up close, with its challenges and issues and how deep everything was,” she said. “Being able to play the guinea pig for such an important science has been very motivating for me.”
And among her peers, she unexpectedly became a model. “Most of my friends, they were a little shocked when I told them what I had planned to do,” she said. They couldn’t believe her parents would leave her. It seemed so scary, they said.
“They were pretty freaked out… but when I explained why I wanted it, they really supported me,” she said. “Some children even contacted me. They think it’s really important and they want to do it too.
::
When I first heard about Stepenosky two weeks ago, I admired his ardor, but considered his confidence a little cavalier. Wasn’t her battle with cancer enough? Why risk your health again for something that is not only unproven, but shrouded in suspicion?
Despite the scientific consensus that a vaccine is the only way to free us from e-learning, face masks, closed businesses and social distancing, only about half of Americans say they intend to be vaccinated. once a vaccine is approved. That’s down from over 70% on polls in May.
And that means the problem is people like me who don’t always trust Big Pharma and don’t believe that advances in medicine will be distributed fairly.
Almost three-quarters of skeptics surveyed linked their reluctance to concerns about side effects and questions about a vaccine’s effectiveness. Others don’t trust the process, given how the virus has been politicized. They fear that a vaccine will be rushed to the market to cause political sensation.
For some, this is a reason to exclude participation in trials. But it was also what helped persuade Stepenosky to take a chance.
“A big part of it is that there isn’t a lot of trust because of the president,” he said. “When politicians lose the trust of the people, we are in trouble. We need to test these vaccines. We cannot wait.
But he also has a practical reason to become a pioneer in testing. Stepenosky is superintendent of the 11,500-student unified school district in Las Virgenes, on the southwestern edge of the San Fernando Valley. On Monday, her district became the first in Los Angeles County to allow elementary school students to return to cleaned and disinfected classrooms. Based on this progress, protective measures such as vaccines will be needed.
“We need to build confidence in the trials and let everyone know that people testing these vaccines are doing well,” Stepenosky said.
There are dozens of similar trials, at different stages, going on around the world. And while preliminary results from the Pfizer trial suggest it is headed for success, the trial won’t end until researchers are able to measure the vaccine’s long-term effects, a declared Stepenosky.
“I think they’re going to stay there. I don’t think it’s a horse that crosses the finish line, and they win and everyone goes.
This is a battle that whether we like it or not we are all going to be in for who knows how long. And we must be grateful not only for the ranks of essential workers, but also for the ordinary people who are ready to be pushed and pushed, and to take the risks therein, so that we can finally venture out again. .
[ad_2]
Source link