[ad_1]

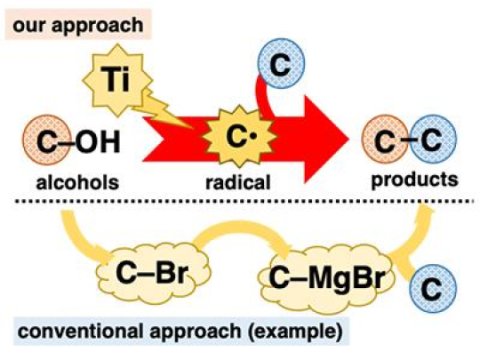
This is a direct use of alcohols for C-C bond formation reactions.
Credit: Kanazawa University
Alcohols play a crucial role in organic synthesis because they are ubiquitous and can be used in various well-established transformations. However, in C-C bond formation reactions, although they are at the heart of organic synthesis, the alcohols are mainly used indirectly. Many alcohol-based reactions require tedious pre-transformation of the hydroxy group (C-OH) to other functional groups such as halogen atoms (eg, C-Br) before the formation of the DC link. The development of one-step C-C bond formation reactions with the aid of alcohols is highly desirable as it allows the application of ubiquitous materials without the expense of a multistep procedure. One way to accomplish this goal is to directly convert the alcohols to known reactive intermediates that undergo instant C-C bond formation reactions. We thought we could achieve this by using low-valence titanium-based reagents. The low valence titanium is a one-electron reducer and a highly oxophilic species. Due to these characteristics, low valence titanium is expected to extract an oxygen atom from the alcohol, cutting the CO bond into an electron reduction for one. generate the corresponding carbon radical (C *). The carbon radical is an extremely reactive intermediate that readily undergoes various reactions, including formation of C-C bonds.
Treatment of 2-naphthalenemethanol with a low valence titanium reagent gave a mixture of two C-O cut products from hydrogenation and dimerization. These reactions themselves were not very useful. however, they were both evidence of the generation of benzyl radical species. With this preliminary result, we expected that the addition of radical scavengers would provide the coupling products between the benzyl radical and the scavengers, thereby interrupting the hydrogenation and dimerization reactions. Indeed, the addition of acrylonitrile as a scavenger gave the coupling product between the benzyl radical and acrylonitrile as the predominant product. The best result was obtained when the low valence titanium reagent was prepared from TiCl4 (collidine) and manganese powder. This alcohol-based C-C direct bond formation reaction has been successfully applied to a series of benzyl alcohol derivatives. Remarkably, the two benzyl alcohols with electron donating and withdrawing substituents on the aromatic ring were suitable for this reaction. In addition, in addition to primary alcohols, secondary and tertiary alcohols were also suitable despite the considerable increase in steric hindrance. Several electron-deficient alkenes other than acrylonitrile were also good reagents. From a practical point of view, this reaction is economical and easy to carry out, at least on a laboratory scale. TiCl4 (collidine) is stable during storage, tolerates brief exposure to air and costs only 10 JPY / mmol.
The importance of this method is that it allows the direct use of alcohols as a radical equivalent of carbon. We have linked ubiquitous alcohols to accumulated knowledge about radical reactions. We believe that this work will spur research into other radical alcohol-based reactions in the near future.
Source:
Kanazawa University. .
Journal reference:
- Takuya Suga, Shoma Shimazu and Yutaka Ukaji. Titanium-Mediated Radical Conjugate Addition Using Benzyl Alcohols As Sources Of Benzyl Radicals. Organic Letters2018; 20 (17): 5389 DOI: 10.1021 / acsorglett.8b02305
Quote this page:
Kanazawa University. "Alcohols as precursors of carbon radicals". November 2, 2018.
Kanazawa University. (2018, November 2). Alcohols as precursors of carbon radicals. . Retrieved November 2, 2018 from www..com / releases / 2018/11 / 181102095507.htm.
Kanazawa University. "Alcohols as precursors of carbon radicals". www..com / releases / 2018/11 / 181102095507.htm (accessed November 2, 2018).
Source link