
[ad_1]
The approval by the US Food and Drug Administration of the first medical device of this type for the treatment of ADHD in children aged 7 to 12 years raises "cautious optimism" on the part of parents and lawyers.
The new device, called the Monarch eTNS system, will cost parents about $ 1,000 and is not yet covered by insurance. It is the size of a mobile phone and works by attaching a small patch to the patient's forehead. It provides low-level stimulation to the parts of the brain that are thought to be involved in ADHD.
"This new device offers a safe, non-drug option for the treatment of ADHD in pediatric patients through light nerve stimulation, a first of its kind," said Carlos Peña, Head of Physical Medicine Device Division. and neurological of the FDA.
In a four-week clinical trial of 62 children with moderate-to-severe ADHD, there was a "statistically significant improvement" in reducing their symptoms. But some doctors, cautiously optimistic, are asking for more research before parents start buying these devices.
"The study relies on a very small sample," said Dr. Max Wiznitzer, co-chair of the professional advisory board for children and adults with ADHD. "Most of the known ADHD medications – Ritalin, Adderall – have a much larger sample and so one study is not enough to cite the effectiveness."
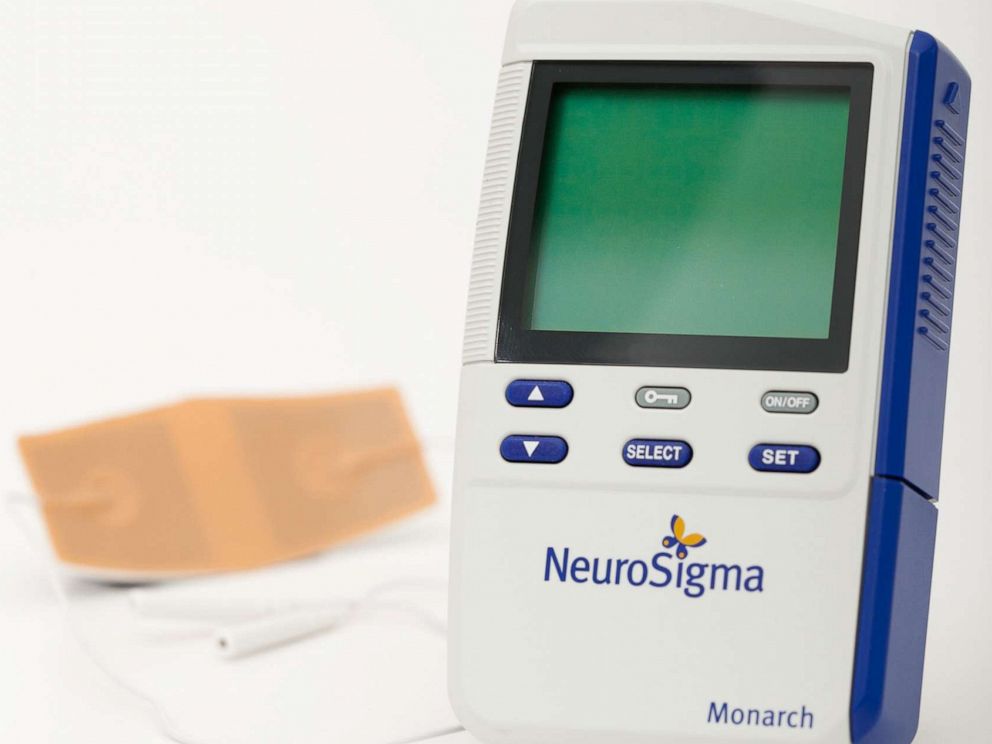
NeuroSigma Inc.
But the known effects of these medications include stomach upset, decreased appetite nervousness and sleep problems.
However, the new Monarch eTNS device does not go without side effects, including drowsiness, headaches, and increased appetite.
"This will probably be a good treatment for children with moderate symptoms of ADHD," said Dr. Wiznitzer. "But my question is whether this device alone will suffice."
Dr. Andy Leuchter, director of clinical research and transcranial magnetic stimulation research at UCLA, participated in the research phase of the device's clinical trial. Dr. Leuchter believes that parents can trust the Monarch eTNS system, but adds that further research, including potentially associating them with other drugs known to treat ADHD, would be helpful.
"It's as close to a treatment with no side effects as you'll get," he said. "So there is very little downside risk to see if there is a benefit."
But even for parents who are struggling to help their children deal with the effects of their own ADHD medications, the device is uncomfortable.
Andrea Frank is the mother of two teenagers in Lodi, Wisconsin, both of whom were diagnosed with ADHD at the age of four, but who have now opted out of treatment. She has created a virtual support group for parents of children with ADHD, which has more than 40,000 members worldwide.
"Personally, I would not go that way," she said. "I see a lot of frustrated parents who feel like they're hitting a wall, but because it's an electrical device, they're afraid to use it."
[ad_2]
Source link