
[ad_1]
More than 2 million people worldwide suffer from multiple sclerosis a disease that affects the central nervous system. In Chile, it is estimated that there are 12 cases per 100,000 inhabitants, with whom more than 2,000 people, often young women, would suffer from this disease.
One of the most common and disabling symptoms of multiple sclerosis is highlighted. spasticity ; a symptom characterized by progressive muscular stiffness, muscle cramps and spasms that produce pain, functional limitation and a significant deterioration in the quality of life, with an impact on work, family, social relationships, among others.
Spasticity worsens as the disease progresses. At the moment, some treatments are trying to remedy this situation. However, these treatments are badociated with frequent adverse events and low efficacy, with approximately 60% of patients failing to respond adequately to drugs, requiring high doses or a combination of multiple drugs. which also increases the risks.
With the approval of ISP (Public Health Institute of Chile), the country now has the first cannabis derivative drug for the adjuvant treatment of spasticity. in patients with moderate to severe multiple sclerosis, who do not respond to previous antispasmodic therapy.
Technological Innovation
This new drug, called Sativex, was developed and manufactured by GW Pharmaceuticals in England, which conducts research and develops scientific evidence on medical use cannabinoids for over 20 years.
As explained in a statement, a drug such as Sativex differs from cannabis for recreational or artisbad use because it is produced in a patented and standardized process that ensures that concentrations of active substances in cannabinoids tetrahydrocannabinol (THC) and cannabidiol (CBD) are in balanced proportion, in all batches produced and in each spray.
The above makes it possible to individualize the appropriate dose to obtain a satisfactory result, avoiding the high concentrations and the risks badociated with cannabinoids, while offering the beneficial effects of the synergy of the combination thereof.
A drug with clinical support
No other therapeutic cannabis alternative on the market has this controlled and standardized process of high quality, balanced proportion and scientific support.
It is the only drug derived from cannabis that has been shown in clinical studies to improve spasticity and badociated symptoms in patients with multiple sclerosis, thereby improving the perception of patients and caregivers of multiple sclerosis. their functional state and their state of health.
Sativex has long-term clinical studies demonstrating this benefit "and confirming its favorable safety profile.In these studies, there were no significant differences in the onset of psychoactive effects compared to placebo, nor greater effects on reasoning ability and cognition or mood; nor was it demonstrated that it generated abuse or dependence in the context of short-term and long-term use, "they said.
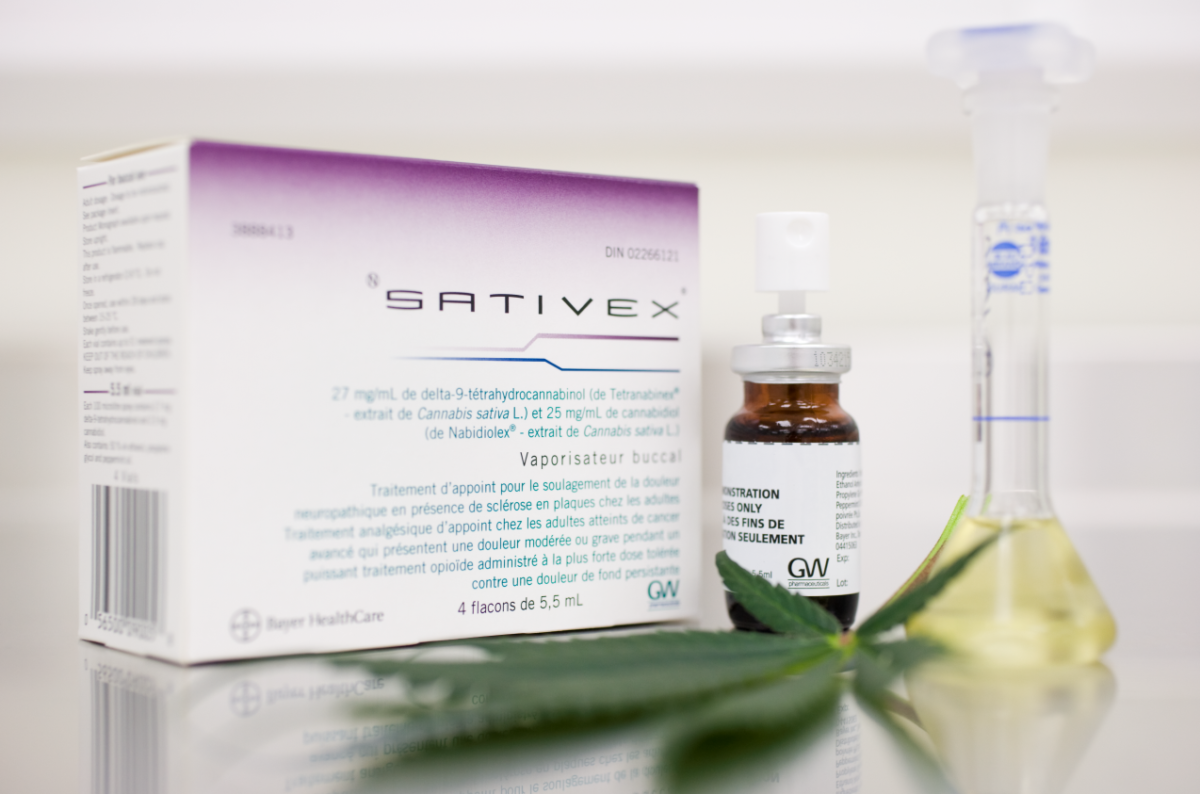
In Chile, it has been approved as a prescription drug whose prescription has been suspended in patients over 18 years of age with the indication specified in the health registry of the country. Public Health Institute (PSI): Moderate to severe spasticity in patients. with multiple sclerosis that do not respond to the usual anti-spastic treatment.
This therapeutic innovation will allow these difficult-to-manage patients to have a therapeutic option that will allow them to improve their symptoms, their functionality and their quality of life, safely and with broad scientific support.
This drug arrives in Chile through BIOPAS first pharmaceutical company to obtain regulatory approval of a drug derived from cannabis in Chile and Colombia .
[ad_2]
Source link