
[ad_1]

The opioid addiction crisis experienced in the United States did not prevent the authorities of this country from authorizing the entry of a new drug of this type called on the market. Dsuvia, ten times more powerful than fentanyl and a thousand times stronger than morphine.
The Food and Drug Administration (FDA) endorsed Dsuvia last Friday, although some Democratic senators and the chair of the FDA's Anesthetic and Analgesic Drugs Advisory Committee, Raeford Brown, had asked not to do so .
The excessive use of opiates has caused a serious crisis in this American nation, where more than 115 people die each day of overdoses . according to data from Centers for Control and Prevention of Disease (CDC). These include painkillers, heroin and synthetic opiates such as fentanyl.
The opioid abuse has not only caused a health crisis, but also an economic crisis, to the extent that they generate a "burden" of […] US $ 78,500 million a year on medical care, lost productivity, drug treatment and court proceedings, according to the CDC.
FDA Commissioner Scott Gottlieb admitted in a statement Friday that opioid addiction is a priority for your institution. But he also indicated the reasons why the FDA authorized its marketing, being the main one among them its military application .
"Priority to the Pentagon"
Dsuvia is a "priority drug for the Pentagon", according to Gottlieb
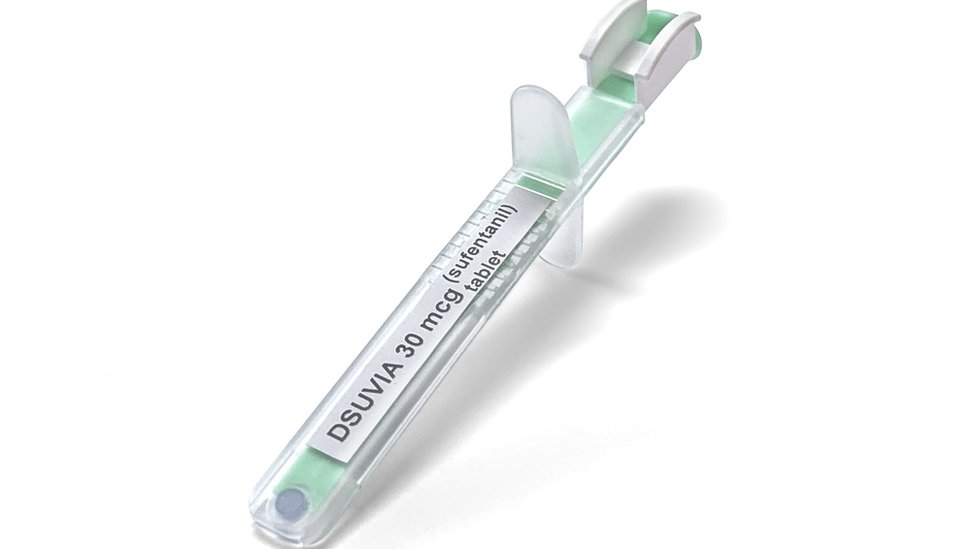
The US military was interested in this drug not only because of its effectiveness, but also because of its form of application .
It was composed of sufentanil, a substance that was administered until now by intravenous or epidural. But the novelty of Dsuvia is that it consists of a small pill that is placed under the tongue with a single-dose applicator.
"These unique features in which the drug is stably administered make it perfectly suited to certain special circumstances patients are not able to swallow oral medications and for which Access to intravenous badgesics is impossible "said Gottlieb.
"This includes possible uses in the battlefield", where this product will meet an "unmet medical need", which allowed the Department of Defense to "work closely together" with the developers of Dsuvia, explained the commissioner.
Gottlieb admitted that in this case, "the military application of this new drug has been carefully considered" and that the needs of the military sector and the involvement of the Defense in the creation Dr. Suvia was part of the debate organized by the FDA Advisory Committee, which decided on October 12 to approve the approval of this drug by 10 votes to 3.
Deviant Use
Raeford Brown , who is a professor of anesthesiology at the University of Kentucky and believes that sufentanil is an "extremely divertable" drug.
This was explained in a letter signed with experts from the Public Citizen lobby group in which he urged the FDA not to give Dsuvia the green light.
"It is a potent opioid with significant risks of respiratory depression, deviation (from its use), abuse and death."
"It is so powerful that those who abuse its intravenous version die often by injecting the first dose, "a situation he claims to have witnessed.
The expert predicts that, even in small formats, in the months following his entry into the market, " will find deviations (in relation to its use), abuse and death ]. "
Gottlieb however recalled in his statement that the European Medicines Agency also approved the same drug in July, although he called Dzuveo.
In addition, she stated that access to Dsuvia would be limited to "sanitary environments with certified medical supervision", such as hospitals or emergency centers. at; It can only be administered by health professionals and never more than 72 hours.
Brown, however, baderted that the FDA "traditionally lacks the ability" to "apply controls" and that sublingual sufentanil "is a danger to public health in general."
"This will make our protection work Americans more difficult. "
You can now receive notifications from BBC News World. Download the new version of our application and activate them to not miss our best content.
Source link