
[ad_1]
In this March 2021 photo provided by Pfizer, vials of the Pfizer-BioNTech COVID-19 vaccine are being prepared for packaging at the company’s facilities in Puurs, Belgium. Many Utahns who have not received a COVID-19 vaccine say the potential side effects have made them hesitate. (Pfizer via AP)
SALT LAKE CITY – If you are one of the millions of Americans who have been vaccinated against COVID-19, you may have experienced minor side effects: headache, chills, fever, and body aches. These reactions were common and well documented. But concerns about more serious side effects prevent many people from getting the vaccine.
The US Census Bureau began keeping tabs on vaccine reluctance through its Household Pulse Survey in July. In its data release for August 4-6, 428,944 Utahns were surveyed. Of these, 53.2% (+/- 10.2%) indicated “being concerned about side effects” as the reason they were “definitely not” or “probably not” receiving a COVID-19 vaccine.
These hesitations increased over the next two weeks.
Between August 18 and 30, 314,826 Utahns were interviewed. This time, 63.1% (+/- 10.1%) cited side effects as a reason for not getting the vaccine.
The Pfizer COVID-19 vaccine received full approval from the United States Food and Drug Administration within the timeframe of this most recent investigation.
Serious side effects reporting and investigation
Many of the online side effect issues relate to data found in the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System (VAERS).
This is a site where anyone can report negative reactions they think are caused by any vaccine. VAERS is an important tool. Reports can help provide an early warning of a vaccine-related safety issue. But think of it like a social network: anyone can post, but that doesn’t mean the information has been verified or is in fact linked to a vaccine.
An important disclaimer on the website’s landing page states, in part, that “VAERS reports alone cannot be used to determine whether a vaccine has caused or contributed to an adverse event or disease. Reports may contain incomplete, inaccurate, incidental, or unverifiable information. For the most part, reports to VAERS are voluntary, which means they are subject to bias. “
“Basically, this is a national early warning detection for all vaccines available in the country,” said Rich Lakin, director of immunization at the Utah Department of Health. “It’s self-reported, so it means someone can self-report it, it means a doctor can report it. So really, it’s just a way for the CDC to look at the data and to see if there are any adverse events occurring. “
CDC considers this information to be so important that users must agree to understand the disclaimer and limitations twice before they can access VAERS datasets.
Lakin told KSL-TV that they receive weekly reports of vaccine side effects from respondents in Utah via VAERS. He said that despite the limitations, the reported VAERS events are useful.
“If there is a serious adverse reaction to the vaccine, then we can examine it further with the doctor,” he explained.
So far, Lakin said they haven’t had to investigate serious cases in Utah. “We have had no deaths in the state of Utah from vaccines (COVID-19),” he said.
According to a joint study by doctors from Johns Hopkins, CDC, FDA and Vanderbilt University, only three of the 7,653 deaths reported to VAERS have been confirmed to be plausibly caused by the Johnson & Johnson COVID vaccine. 19. In these cases, the deaths are due to a rare disease of blood clotting with a low number of platelets.
Whatever the limitations of VAERS, reports are useful to public health officials.
As of September 10, 3,665 Utah residents have reported COVID-19-related adverse events to VAERS. There are 17,525 events, which can include several side effects for a person reporting.
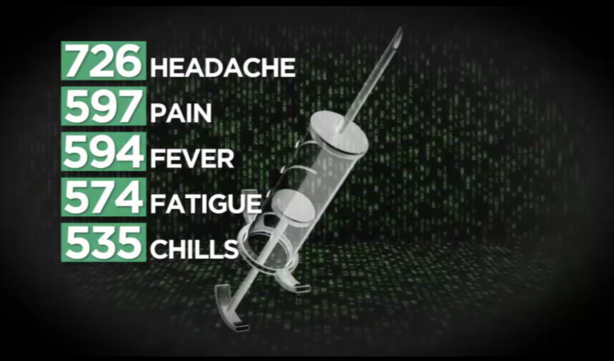
The five most common side effects listed for Utahns include:
- Headache (726 events)
- Pain (597 events)
- Fever (594 events)
- Fatigue (574 events)
- Chills (535 events)
All of these effects have been widely reported by people receiving one or more doses of the vaccine. Conditions or side effects that the CDC deemed “serious” were much less common. In an email, the CDC told KSL these would include death, life-threatening illness, hospitalization or extended hospitalization, permanent disability, birth defects or birth defects.
In Utah, 6.93% of people reported serious side effects to VAERS.
Nationally, 42,211 of 497,262 event reports, or 7.82%, were considered serious.
According to Bloomberg, 382 million vaccines have been administered in the United States as of September 15. Using this number, reported serious side effects represent 0.01105% of all vaccines.
So how does the CDC determine if the vaccine caused these more serious problems?
KSL investigators have asked the CDC what steps they are taking to verify the VAERS reports. In an email, his spokesperson told us that anything classified as a serious event is under investigation. They “request and examine all available medical records: hospital records, clinical records, death certificates and autopsy reports to better understand the adverse event.”
So far, VAERS reports have shown a few patterns that the CDC is closely monitoring and researching when it comes to COVID-19 vaccines. These include:
- Anaphylaxis or severe allergic reactions.
- Thrombosis with thrombocytopenia syndrome – a rare disease of blood clots with a low number of platelets.
- Guillain-Barré syndrome – a rare condition in which the body’s immune system damages cells in the nervous system, causing muscle weakness and sometimes paralysis.
- Myocarditis and pericarditis – inflammation of the heart muscle and the thin layer of tissue around the heart.
Effects of COVID-19 on the heart
The CDC said myocarditis and pericarditis mostly occur in adolescent men and young adults. As of September 8, VAERS had received 1,413 reports of these illnesses in people aged 30 and under.
Its website reports that “the CDC and the FDA have confirmed 854 reports of myocarditis or pericarditis” and “are investigating” to see if they are related to the COVID-19 vaccine.
“These cases are, and certainly should be, reported,” said Dr Damian Domanski, cardiologist at Lone Peak Hospital.
Domanski reported that his practice has seen around eight to 10 people with vaccine-related myocarditis, which he is required by law to report to VAERS.
“Fortunately, an excellent majority of them have very mild symptoms that go away within days or even weeks,” said Domanski. “Yes, there are patients whose symptoms are more prolonged, indeed, but luckily none of these patients develop real significant damage to the heart or have sustained permanent damage.”
Statistically, about five to six in 100,000 people had myocarditis after the vaccine. This is compared to 150 in 100,000 who contract myocarditis as a result of contracting the COVID-19 virus.
“COVID significantly affects the heart more often than the vaccine itself,” explained Domanski. “I have seen a lot of patients here with serious heart complications from COVID infection. Unfortunately, some of these patients have died.”
At the end of the day, Lakin encouraged anyone receiving a vaccine that causes side effects – even minor ones – to report them to VAERS for analysis and investigation, saying that “all of these things ensure that we can monitor and make sure that there is Nothing extraordinary. “
He and Domanski encouraged anyone who still has doubts about COVID-19 vaccines and possible side effects to speak with a trusted family doctor.
Related stories
More stories that might interest you
[ad_2]
Source link

