[ad_1]

Among the 100 million people around the world who have battled coronavirus infections, scientists are looking to the case of a 45-year-old COVID-19 patient in Boston to understand how the virus is able to outsmart humans.
During his 154-day illness – one of the longest on record – the patient’s body became a melting pot of rioting viral mutations. It offered the world one of the first observations of a key mutation in the virus spike protein that raised the alarm when it was later found in strains in the UK, South Africa and in Brazil.
In the British strain, the genetic change known as N501Y is believed to help improve the transmissibility of the virus by about 50%. In the South African strain, this may reduce the effectiveness of COVID-19 vaccines and treatments. Tests of its effect on the Brazilian variant are still ongoing.
The Boston patient is now seen as an important harbinger of the coronavirus’s ability to create new and more dangerous versions of itself. Although he passed away over the summer, the medical records he left behind are helping experts anticipate the emergence of new strains by focusing on the role of a growing population of immune-system patients. weakened who fight the virus for months.
Among the sickest COVID-19 patients, this “long haul” population appears to play a key role in incubating new variants of the coronavirus, some of which could change the trajectory of the pandemic.
The mutations that arose from this single patient are “a microcosm of the viral evolution that we see around the world,” said Dr. Jonathan Z. Li, an infectious disease specialist at Brigham and Women’s Hospital in Boston who treated. “He showed us what could happen” when a germ with a talent for genetic shapeshifting comes across conditions that reward it for doing so.
Indeed, situations where patients cannot rule out a viral infection are “the worst possible scenario for developing mutations,” said Dr. Bruce Walker, immunologist and founding director of the Ragon Institute in Boston.
As sick weeks turn into months, a virus copies itself millions of times. Every copy is an opportunity to make random mistakes. By creating new mutations, the virus can occur on those that help it resist drugs, evade the immune system, and come back stronger.
SARS-CoV-2, the coronavirus responsible for COVID-19, has been an unpredictable adversary. The chance to witness its transformation in near real time and see where and how it mutates in a single host can guide the design of vaccines and drugs that do not lose their effectiveness over time, Walker said.
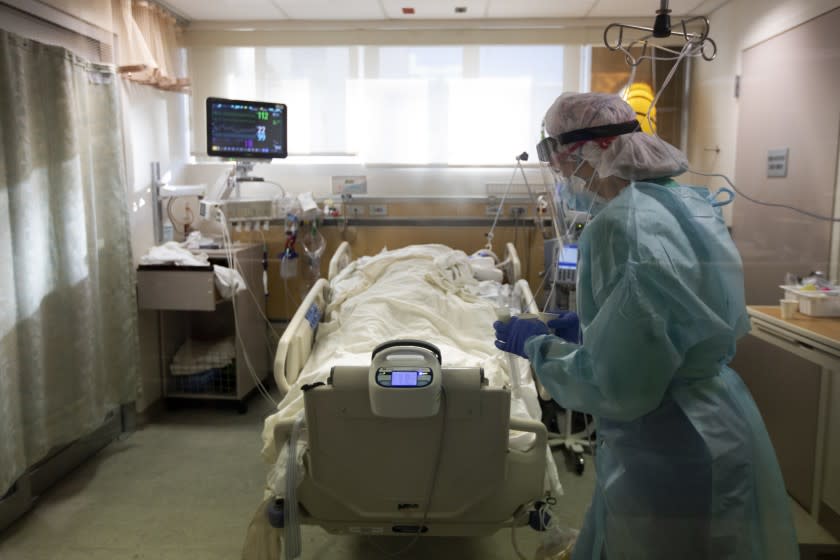
COVID-19 patients were just starting to fill beds at Brigham and Women’s Hospital in the spring of 2020, when the Boston patient was first admitted. He had fever, nausea, and a CT scan of his lungs that bore the hallmark appearance of the new disease, said Li, who was part of a team that detailed the man’s case in the New England Journal of Medicine.
But COVID-19 was only one of his challenges. For 22 years he suffered from a rare disease called antiphospholipid syndrome, which caused his immune system to attack his own organs and cause dangerous blood clots throughout his body.
To prevent his rogue immune system from killing him, the patient needed an arsenal of immunosuppressive drugs. But in his fight against the coronavirus, these drugs kept the patient’s arm tied behind his back.
The Boston patient tested positive for SARS-CoV2 infections four different times over 22 weeks. He has been admitted to hospital six times, including intensive care stays. Doctors treated him with three courses of the antiviral drug, remdesivir, and once with Regeneron’s experimental monoclonal antibody cocktail.

Swabs taken from his nose and throat during his second hospital stay provided the first clue of the astonishing rate of genetic transformation of the virus: compared to a sample taken during his first hospitalization, 11 letters in the sequence of 30,000 letters of the coronavirus had switched, and nine of those nucleotides had given up.
His next trip to the hospital took him to the ICU. Tests revealed that 10 more letters of the virus’s genetic code had changed and another was deleted in just five weeks. Three weeks later, after appearing to be healing, he tested positive again and was put on a mechanical ventilator to help him breathe. This time, the researchers found 11 letter changes and 24 additional deletions in the virus genome.
Scientists couldn’t tell if the Boston patient was failing to clear the virus or if it changed so completely that his immune system couldn’t recognize him.
One thing was clear: more than half of the alterations occurred in a stretch of genetic code that dictates the structure of the virus’s spike protein, the protuberance that clings to human cells and initiates infection. The virus’ “receptor binding domain” – essentially the key that locks a human cell – makes up only 2% of the virus’s genetic code. But 38% of the mutations caused during the prolonged illness of the Boston patient were concentrated there.
In late December, British scientists hypothesized that such a scenario involving an immunocompromised patient somewhere in England could have given rise to the mutations that distinguish the British strain.
Walker said he feared there would be many more such patients, including those with untreated HIV. Immunosuppressed by HIV, suffering from COVID-19 and receiving drugs that reward SARS-CoV-2 for inventing “escape” mutations, these people could become crucibles of viral mutations.
Scientists in South Africa share this concern.
“In South Africa, the country with the largest HIV epidemic in the world, a concern has been prolonged viral replication and intra-host evolution in the context of HIV infection,” the authors wrote. a preliminary study that alerted the world to the new variant. early December.

So far, there is no evidence that HIV-positive patients are more prone to long-lasting cases of COVID-19. And even if they were, a long chain of immunocompromised patients would likely have been required to generate the many mutations that distinguish the South African strain, its discoverers said.
Scientists are still trying to figure out how certain mutations like N501Y appeared in many places at once. Has the growing scale of the pandemic given the virus too many opportunities to change? Or do these mutations occur in a small number of people, like the patient in Boston, and then hitchhike around the world?
Both factors are likely at work, and the longer and hotter the pandemic, the more likely the virus will be to develop random mutations.
The Boston patient shows why it can be so dangerous. In his case, the segments of genetic code most likely to change the affected structures that COVID-19 vaccines and drugs are designed to recognize. There are now signs that the changes could undermine the value of these remedies.
Tulio de Oliveira, an infectious disease researcher at the University of South Africa of KwaZulu-Natal, sees a model in which uncontrolled spread and long-distance infections work in tandem to fuel mutations in the coronavirus.
Many places where new variants have been identified – including South Africa, Britain and California – have seen two waves of divided epidemics in just a few months. This, De Oliveira suspects, is no mere coincidence.
During the first wave, he said, the proliferation of infections gives the virus many opportunities to take charge of genetic changes that may linger in the bodies of immunocompromised patients. By the time a second wave begins, new variants that were incubating among these long haul also began to circulate. When they encounter large numbers of new hosts, the result is a fertile environment for strains to establish – if their genetic modifications give them an advantage.
The best way to prevent more mutations from emerging is to both expand vaccinations and do more to protect people with weakened immune systems, De Oliveira said.
“If we keep the virus for a long time, we will give it more opportunities to outsmart us,” he said.
This story originally appeared in the Los Angeles Times.
[ad_2]
Source link