[ad_1]
Family medicine doctor Mikhail Varshavski gives an overview of coronavirus vaccine trials.
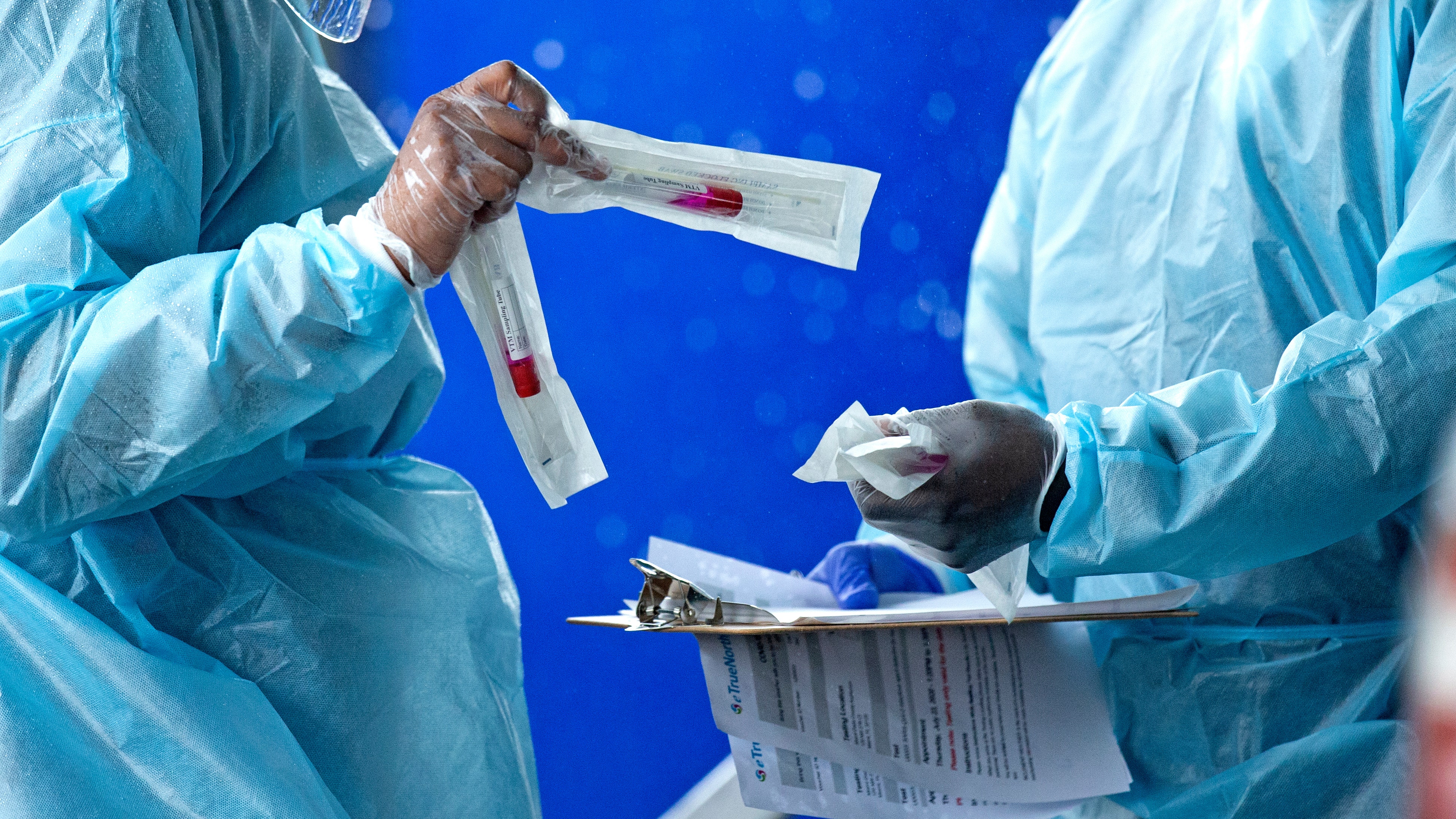
(Reuters) – The U.S. Food and Drug Administration said on Tuesday it had approved the first COVID-19 home-use self-test kit that provides results in 30 minutes.
LISTERINE SAYS IT IS NOT CLINICALLY PROVEN TO FIGHT COVID-19
The single-use test, performed by Lucira Health, received emergency use authorization for home use with self-collected nasal swab samples from people aged 14 and over suspected of COVID-19 by their health care provider, the FDA said.
“While the COVID-19 diagnostic tests have been authorized for home collection, it is the first that can be fully self-administered and provide results at home,” said FDA Commissioner Stephen Hahn.
The kit can also be used in hospitals and point-of-care, but samples must be collected by a healthcare provider if those tested are under 14, the health regulator said.
MODERNA CEO WELCOMES CORONAVIRUS VACCINE SAFETY AS ‘GAMECHANGER’, WITH 20M DOSES AVAILABLE BY YEAR END
Although a recent spate of positive news from Moderna Inc and Pfizer Inc on their potential vaccines has raised hopes in the fight against the disease, testing is still a key factor in controlling the spread of the virus.
“We look forward to working proactively with test developers to support the availability of more home testing options,” said Jeff Shuren, director of the Center for Devices and Radiological Health at the FDA.
On Sunday, the United States crossed 11 million infections in total, just eight days after hitting the 10 million mark.
CLICK HERE TO LEARN MORE ABOUT FOX BUSINESS
[ad_2]
Source link